Distribution characteristics of arsenic in sediments and its control on groundwater arsenic enrichment : A case study of Hetao Basin, Inner Mongolia
-
摘要:
研究目的 河套盆地西侧存在原生高砷地下水,查明含水层沉积物中砷的空间分布和赋存特征,分析地下水砷的富集机理,有利于保障当地居民用水安全。
研究方法 本研究在河套盆地山前冲洪积扇区的钻孔K02和河套盆地平原区的钻孔K01中分别采集25和26个沉积物样品,用于分析岩性特征与地球化学组分,并开展砷的分步提取与解吸附实验。
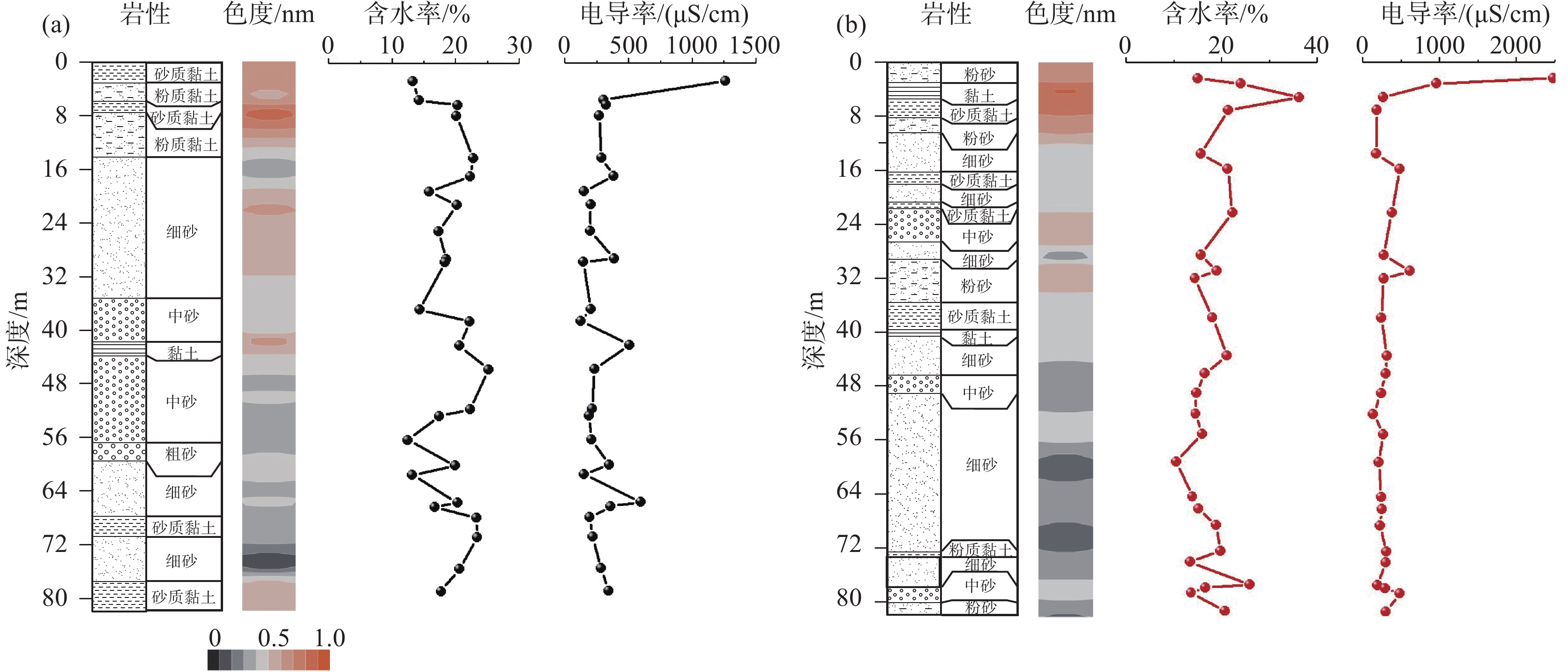
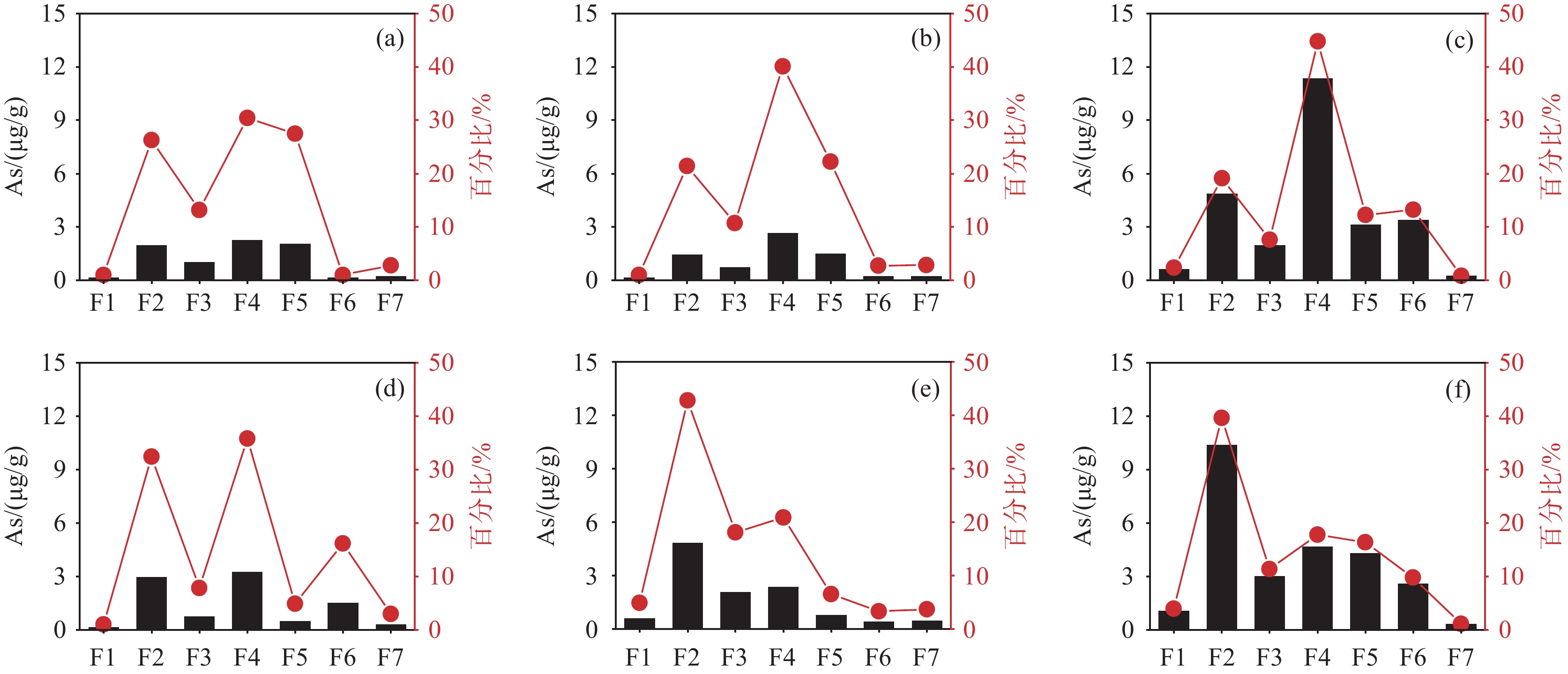
研究结果 山前冲洪积扇区含水层处于相对氧化的环境中,而平原区含水层处于封闭的还原环境中。后者沉积物电导率普遍高于前者,两者沉积物电导率随深度均有递减趋势。山前冲洪积扇区和平原区沉积物总固相砷含量相差不大,但固相砷的赋存形态差别较大,前者沉积物固相砷以无定形态的铁氧化物或氢氧化物共存的砷为主,后者则以强吸附态砷为主。
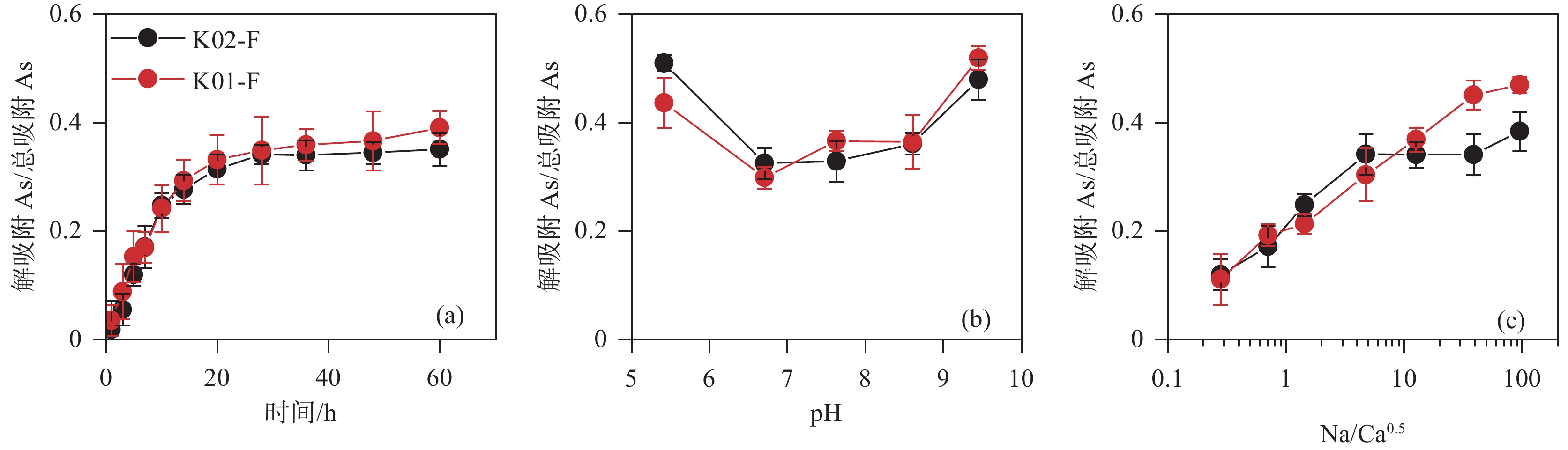
结论 沉积物中砷赋存特征的差异是造成平原区地下水砷浓度高于山前冲洪积扇区的主要原因。解吸附实验表明弱碱性环境或高Na/Ca0.5摩尔比值均能促进砷的解吸附,导致地下水中砷的富集。
Abstract:This paper is the result of hydrogeological survey engineering.
Objective Higharsenic (As) groundwater occurred in the west of Hetao Basin. Investigating the spatial distribution and occurrence characteristics of As in aquifer sediments and studying the enrichment mechanism of As ingroundwater are beneficial to ensure the safety of water for local residents.
Methods Twenty five sediment samples from borehole K02 in the piedmont alluvial fan and twenty sixsamples from borehole K01 in the plain were collectedto analyze lithological characteristics and geochemical components. These samples were further to conduct sequential extraction and desorption experiments of As in sediments.
Results The aquifers in the piedmont alluvial fan were in a relatively oxidized environment, while the aquifers in the plain area were in a closed reducing environment. Salinity of the latter sediment was generally higher than that of the former, and salinity of both sediments had a decreasing trend with depth. Total solid As content in the sediments of the piedmont alluvial vans and the plain area displayed little difference, but the occurrence pool of solid Aswas quite different. The former sediment solid As was dominated by the As incorporated in very amorphous Fe−(oxyhydr) oxides, while the latter was dominated by strongly adsorbed As.
Conclusions The differences of As occurrence characteristics in sediments were the main reason why groundwater As concentration in the plain area was higher than that in piedmont alluvial vans. Desorption experiments showed that weak alkalinity or high Na/Ca0.5 molar ratio could promote the desorption of As.
-
Key words:
- sediment /
- solid arsenic /
- occurrence form /
- hydrogeological survey engineering /
- Hetao Basin /
- Inner Mongolia
-

-
表 1 分步提取实验具体步骤
Table 1. Sequential extraction procedure
步骤 目标物 提取剂 条件 F1 弱吸附态砷 0.05 mol/L (NH4)2SO4 25 mL,25℃,4 h,重复一次,水洗一次 F2 强吸附态砷 0.5 mol/L NaH2PO4 40 mL,25℃,16 h及24 h各一次,每个时间段重复一次,水洗一次 F3 与可挥发硫化物、碳酸盐、锰氧化物和完全无定形态的铁氧化物或氢氧化物共存的砷 1 mol/L HCl 40 mL,25℃,1 h,重复一次,水洗一次 F4 与无定形态铁氧化物或氢氧化物共存的砷 0.2 mol/L NH4H2C2O3 40 mL,25℃,2 h,pH=3,黑暗条件下,重复一次,水洗一次 F5 与结晶态铁氧化物或氢氧化物
共存的砷0.5 mol/L NaC6H8O7
1 mol/L NaHCO3,Na2S2O4XH2O35 mL NaC6H8O7+2.5 mL NaHCO3(加热至85℃),加0.5 g Na2S2O4XH2O,15 min在85℃,重复一次,水洗一次 F6 与硅酸盐有关的砷 10 mol/L HF,H3BO3 40 mL,25℃,1 h、24 h、16 h后各加5 g硼酸,每个时间段重复一次,热水洗一次 F7 含砷硫化物,与硫化物和有机质
共沉淀的砷16 mol/L HNO3,30% H2O2 先加入10 mL HNO3,反应过后加入多次30%过氧化氢,加热,冷却后稀释到100 mL,离心、过滤、测试 表 2 离子强度为(130±5)mmol/L条件下,不同浓度NaCl和CaCl2混合液的Na/Ca0.5(M/M)比值
Table 2. Na/Ca0.5(M/M) ratio of the mixed solution of different concentrations of NaCl and CaCl2 under the condition of ionic strength of about (130±5) mmol/L
NaCl/(mmol/L) CaCl2/(mmol/L) Na/Ca0.5 2 43 0.3 5 42 0.7 10 40 1.6 30 35 5.0 60 23 13 110 7 42 125 1.5 102 表 3 用于分步提取的沉积物信息
Table 3. Sediment information for SEP
编号 岩性 采样深度/m K02−M 中砂 38.35 K02−F 细砂 62.25 K02−C 黏土 41.95 K01−F 细砂 55.15 K01−S 粉砂 30.95 K01−C 黏土 37.85 -
[1] Bélanger N, VanRees K C J. 2007. Soil Sampling and Methods of Analysis, SecondEdition[M]. Crc Press, 15–24.
[2] Cao Wengeng, Wang Yanyan, Ren Yu, Fei Yuhong, Li Jincheng, Li Zeyan, Zhang Dong, Shuai Guanyin. 2022. Status and progress of treatment technologies for arsenic–bearing groundwater[J]. Geology in China, 49(5): 1408−1426 (in Chinese with English abstract).
[3] Cook S J, Levson V M, Jackaman W, Giles T R. 1995. A comparison of regional lake sediment and till geochemistry surveys–a case–study from the Fawnie Creek area, Central British Columbia[J]. Exploration and Mining Geology, 4(2): 93−110.
[4] Cui Xingtao, Wang Xueqiu, Luan Wenlou. 2015. An analysis of modes of occurrence and biological availability of the heavy metal elements in soil of the central and southern plain in Hebei[J]. Geology in China, 42(2): 655−663 (in Chinese with English abstract).
[5] Drahota P, Peřestá M, Trubač J, Mihaljevič M, Vaněk A. 2021. Arsenic fractionation and mobility in sulfidic wetland soils during experimental drying[J]. Chemosphere, 277: 130306. doi: 10.1016/j.chemosphere.2021.130306
[6] Dzombak DA, MorelF M M. 1990. Surface Complexation Modelling–Hydrous Ferric Oxide[M]. John Wiley, New York.
[7] Eiche E, Neumann T, Berg M, Weinman B, van Geen A, Norra S, Berner Z, Trang P T K, Viet P H, Stüben D. 2008. Geochemical processes underlying a sharp contrast in groundwater arsenic concentrations in a village on the Red River delta, Vietnam[J]. Applied Geochemistry, 23(11): 3143−3154. doi: 10.1016/j.apgeochem.2008.06.023
[8] Eiche E, Kramar U, Berg M, Berner Z, Norra S, Neumann T. 2010. Geochemical changes in individual sediment grains during sequential arsenic extractions[J]. Water Research, 44(19): 5545−5555. doi: 10.1016/j.watres.2010.06.002
[9] Fakhreddine S, Dittmar J, Phipps D, Dadakis J, Fendorf S. 2015. Geochemical triggers of arsenic mobilization during managed aquifer recharge[J]. Environmental Science & Technology, 49(13): 7802−7809.
[10] Guo Huaming, Guo Qi, Jia Yongfeng, Liu Zeyun, Jiang Yuxiao. 2013. Chemical characteristics and geochemical processes of high arsenic groundwater in different regions of China[J]. Journal of Earth Sciences and Environment, 35(3): 83−96 (in Chinese with English abstract).
[11] Guo H M, Yang S Z, Tang X H, Li Y, Shen Z L. 2008. Effect of indigenous bacteria on geochemical behavior of arsenic in aquifer sediments from the Hetao Basin, Inner Mongolia: Evidence from sediment incubations[J]. Applied Geochemistry, 23(12): 3267−3277. doi: 10.1016/j.apgeochem.2008.07.010
[12] Horneman A, van Geen A, Kent D V, Mathe P E, Zheng Y, Dhar R K, O’Connell S, Hoque M A, Aziz Z, Shamsudduha M, Seddique A A, Ahmed K M. 2004. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part I: Evidence from sediment profiles[J]. Geochimica et Cosmochimica Acta, 68(17): 3459−3473. doi: 10.1016/j.gca.2004.01.026
[13] Han Shuangbao, Zhang Fucun, Zhang Hui, Jia Xiaofeng, He Jin, Li Xufeng. 2010. An analysis of the distribution and formation of high arsenic groundwater in northern China[J]. Geology in China, 37(3): 747−753 (in Chinese with English abstract).
[14] He Jin, Ma Xuemei, Pang Yajie, Niu Xue. 2020. Comprehensive evaluation system of soil and water quality in typical farming area of the Sanjiang Plain[J]. Geological Survey and Research, 43(3): 271−278 (in Chinese with English abstract).
[15] Keon N E, Swartz C H, Brabander D J, Harvey C, Hemond H F. 2001. Validation of an arsenic sequential extraction method for evaluating mobility in sediments[J]. Environmental Science and Technology, 35: 2778−2784. doi: 10.1021/es001511o
[16] Li Xiaofeng. 2018. Geochemical Characteristics of Sediments in Piedmont Plains of Hetao Basin and its Significance for Controlling Arsenic in Groundwater [D]. Beijing: China University of Geosciences (Beijing), 1–78 (in Chinese with English abstract).
[17] Liu Xinmin. 2014. Soil ion Exchange Balance: Electric Field, Quantum Fluctuations and their Coupling Effects [D]. Chongqing: Southwest University, 1–149(in Chinese with English abstract).
[18] Mandal B K, Suzuki K T. 2002. Arsenic round the world: A review[J]. Talanta, 58(1): 201−235. doi: 10.1016/S0039-9140(02)00268-0
[19] Masue Y, Loeppert R H, Kramer T A. 2007. Arsenate and arsenite adsorption and desorption behavior on coprecipitated aluminum: Iron hydroxides[J]. Environmental Science and Technology, 41(3): 837−842. doi: 10.1021/es061160z
[20] Ma Xuemei, Tian Dazheng, Li Wei, He Jin. 2020. Geochemical evaluation of land quality in Qvyang County[J]. Geological Survey and Research, 43(3): 230−239 (in Chinese with English abstract).
[21] Meharg A A, Scrimgeour C M, Hossain S S, Fuller K A, Cruickshank K, Williams P N, Kinniburgh D G. 2006. Co–deposition of organic carbon and arsenic in Bengal Delta Aquifers[J]. Environmental Science and Technology, 40(16): 28−35.
[22] Neidhardt H, Berner Z A, Freikowski D, Biswas A, Majumder S, Winter J, Gallert C, Chatterjee D, Norra S. 2014. Organic carbon induced mobilization of iron and manganese in a West Bengal Aquifer and the muted response of groundwater arsenic concentrations[J]. Chemical Geology, 367: 51−62. doi: 10.1016/j.chemgeo.2013.12.021
[23] Paul C J, Ford R G, Wilkin R T. 2009. Assessing the selectivity of extractant solutions for recovering labile arsenic associated with iron (hydr)oxides and sulfides in sediments[J]. Geoderma, 152(1): 137−144.
[24] Shen M M, Guo H M, Jia Y F, Cao Y S, Zhang D. 2018. Partitioning and reactivity of iron oxide minerals in aquifer sediments hosting high arsenic groundwater from the Hetao basin, PR China[J]. Applied Geochemistry, 89: 190−201. doi: 10.1016/j.apgeochem.2017.12.008
[25] Smedley P L, Kinniburgh D G. 2002. A review of the source, behaviour, and distribution of arsenic in natural waters[J]. Applied Geochemistry, 17: 517−568. doi: 10.1016/S0883-2927(02)00018-5
[26] van Geen A, Bostick B C, Thi Kim Trang P, Lan V M, Mai N N, Manh P D, Viet P H, Radloff K, Aziz Z, Mey J L, Stahl M O, Harvey C F, Oates P, Weinman B, Stengel C, Frei F, Kipfer R, Berg M. 2013. Retardation of arsenic transport through a Pleistocene aquifer[J]. Nature, 501: 204–207.
[27] van Geen A, Bostick B C, Thi Kim Trang P, Lan V M, Mai N N, Manh P D, Viet P H, Radloff K, Aziz Z, Mey J L, Stahl M O, Harvey C F, Oates P, Weinman B, Stengel C, Frei F, Kipfer R, Berg M. 2013. Retardation of arsenic transport through a Pleistocene aquifer[J]. Nature, 501: 204–207.
[28] Van Herreweghe S, Swennen R, Vandecasteele C, Cappuyns V. 2003. Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples[J]. Environmental Pollution, 122(3): 323−342. doi: 10.1016/S0269-7491(02)00332-9
[29] Wang Y X, Li J X, Ma T, Xie X J, Deng Y M, Gan Y Q. 2020. Genesis of geogenic contaminated groundwater: As, F and I[J]. Critical Reviews in Environmental Science and Technology, 51(24): 1−39.
[30] Wenzel W W, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano D C. 2001. Arsenic fractionation in soils using an improved sequential extraction procedure[J]. Analytica Chimica Acta, 436(2): 309−323. doi: 10.1016/S0003-2670(01)00924-2
[31] World Health Organization. 2017. Guidelines for Drinking–Water Quality, Fourth Edition Incorporating the First Addendum[S]. Geneva: World Health Organization.
[32] Yuan R X, Guo H M, Zhang D, Li Y, Zhang Y L, Cao W G. 2017. Soluble components of sediments and their relation with dissolved arsenic in aquifers from the Hetao Basin, Inner Mongolia[J]. Journal of Soils and Sediments, 17(12): 2899−2911. doi: 10.1007/s11368-017-1770-9
[33] Zhang Wenkai, Yu Kun, Li Yongzhi, Ji Xinyang, Li Dan, Zhao Zheng, Jiang Xiao. 2020. Research progress of groundwater environment in Hetao Plain[J]. Environmental Chemistry, (2): 489−499 (in Chinese with English abstract).
[34] Zhang Z, Guo H M, Liu S, Weng H C, Han S B, Gao Z P. 2020. Mechanisms of groundwater arsenic variations induced by extraction in the western Hetao Basin, Inner Mongolia, China[J]. Journal of Hydrology, 583: 124599. doi: 10.1016/j.jhydrol.2020.124599
[35] Zhang Zhuo, Liu Futian, Chen Sheming. 2023a. Review on the application of H, O, Sr, Ca, Li and B isotopes in the research of high–fluoride groundwater[J]. North China Geology, 46(3): 49−56 (in Chinese with English abstract).
[36] Zhang Zhuo, Liu Futian, Chen Sheming, Niu Xiaotong, Gao Zhipeng. 2023b. Distribution characteristics and formation mechanism of high fluoride groundwater in Luan River Delta and suggestions for its utilization[J]. Geology in China, 50(3): 887−896 (in Chinese with English abstract).
[37] Zhu Danni, Zou Shengzhang, Zhou Changsong, Lu Haiping, Xie Hao. 2021. Hg and As contents of soil–crop system in different tillage types and ecological health risk assessment[J]. Geology in China, 48(3): 708−720 (in Chinese with English abstract).
[38] 曹文庚, 王妍妍, 任宇, 费宇红, 李谨丞, 李泽岩, 张栋, 帅官印. 2022. 含砷地下水的治理技术现状与进展[J]. 中国地质, 49(5): 1408−1426. doi: 10.12029/gc20220504
[39] 崔邢涛, 王学求, 栾文楼. 2015. 河北中南部平原土壤重金属元素存在形态及生物有效性分析[J]. 中国地质, 42(2): 655−663. doi: 10.3969/j.issn.1000-3657.2015.02.023
[40] 郭华明, 郭琦, 贾永锋, 刘泽云, 姜玉肖. 2013. 中国不同区域高砷地下水化学特征及形成过程[J]. 地球科学与环境学报, 35(3): 83−96. doi: 10.3969/j.issn.1672-6561.2013.03.008
[41] 韩双宝, 张福存, 张徽, 贾小丰, 何锦, 李旭峰. 2010. 中国北方高砷地下水分布特征及成因分析[J]. 中国地质, 37(3): 747−753.
[42] 何锦, 马雪梅, 庞雅婕, 牛雪. 2020. 三江平原典型农垦区水土质量综合评价体系研究[J]. 地质调查与研究, 43(3): 271−278.
[43] 李晓峰. 2018. 河套盆地山前平原沉积物地球化学特征及其对地下水砷的控制意义[D]. 北京: 中国地质大学(北京), 1–78.
[44] 刘新敏. 2014. 土壤离子交换平衡: 电场、量子涨落及其耦合作用[D]. 重庆: 西南大学, 1–149.
[45] 马雪梅, 田大争, 李伟, 何锦. 2020. 曲阳县土地质量地球化学评价[J]. 地质调查与研究, 43(3): 230−239.
[46] 张文凯, 于坤, 李勇志, 冀欣阳, 李丹, 赵政, 蒋校. 2020. 河套平原地下水环境质量研究综述及展望[J]. 环境化学, 39(2): 489−499.
[47] 张卓, 柳富田, 陈社明. 2023a. 氢氧、锶钙和锂硼同位素在高氟地下水研究中的应用[J]. 华北地质, 46(3): 49−56.
[48] 张卓, 柳富田, 陈社明, 牛笑童, 高志鹏. 2023b. 滦河三角洲高氟地下水分布特征、形成机理及其开发利用建议[J]. 中国地质, 50(3): 887−896.
[49] 朱丹尼, 邹胜章, 周长松, 卢海平, 谢浩. 2021. 不同耕作类型下土壤–农作物系统中汞、砷含量与生态健康风险评价[J]. 中国地质, 48(3): 708−720. doi: 10.12029/gc20210303
-




 下载:
下载: