HYDROCHEMICAL CHARACTERISTICS OF GROUNDWATER IN KARST MOUNTAINOUS AREAS OF GUIZHOU PROVINCE
-
摘要:
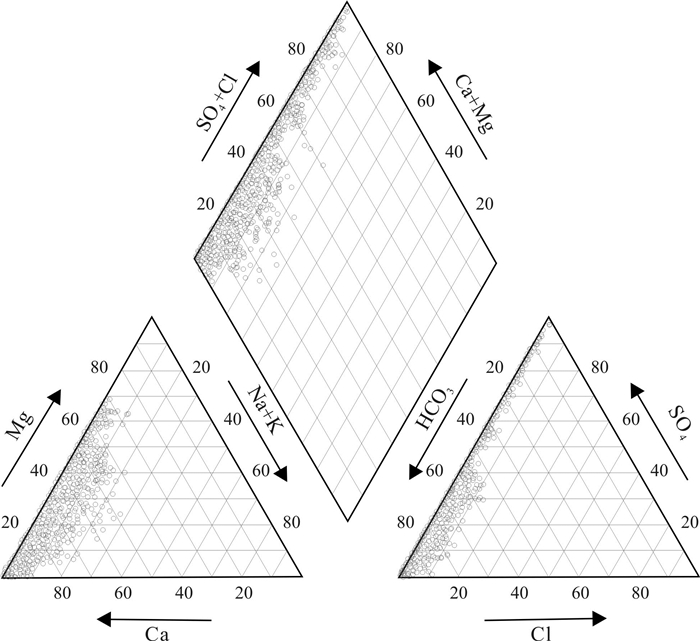
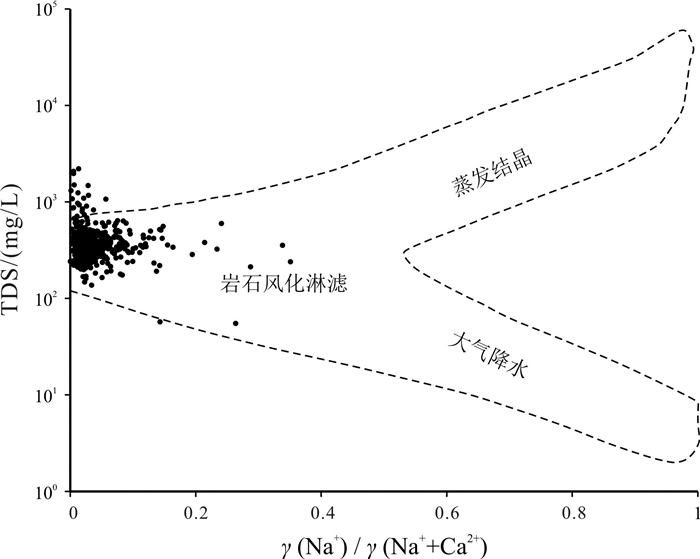
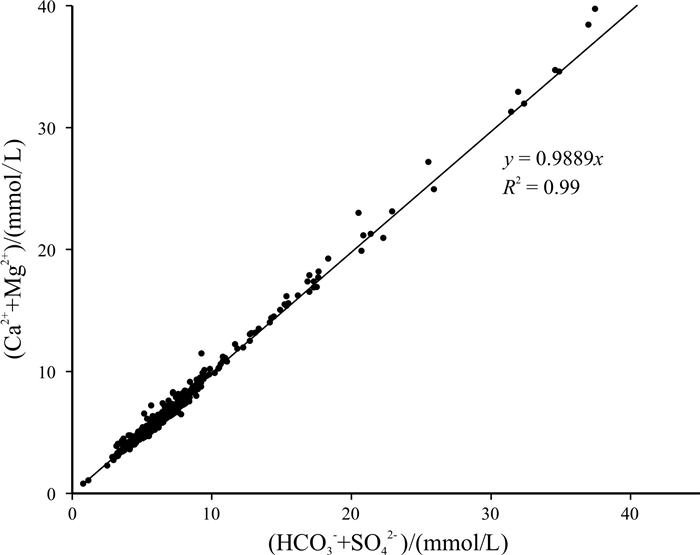
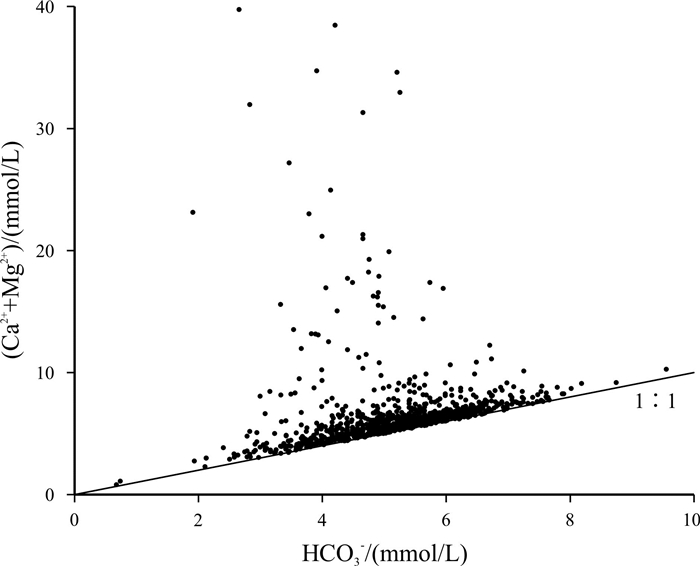
基于贵州省找水打井工程1 067口钻孔的水质检测数据, 运用描述性统计分析、变异系数、Piper图、舒卡列夫分类、Gibbs图及离子比例系数等方法对研究区喀斯特水化学及分布特征进行分析, 并探讨了主要离子成分的来源及其形成作用. 研究结果表明, 研究区地下水中阳离子以Ca2+、Mg2+为主, 阴离子以HCO3-为主, 地下水化学类型主要为HCO3-Ca·Mg型; 地下水化学组分主要来源于碳酸盐岩的溶滤作用, Ca2+、Mg2+主要来自碳酸盐岩矿物的溶解, 且矿物成分对Ca2+、Mg2+含量起到控制作用; Na+、K+的主要来源除受岩盐溶滤作用影响, 还受其他含Na、K类矿物及人为作用的影响, 而阳离子交替吸附作用影响较小; SO42-主要来源于石膏等蒸发岩类的溶滤作用.
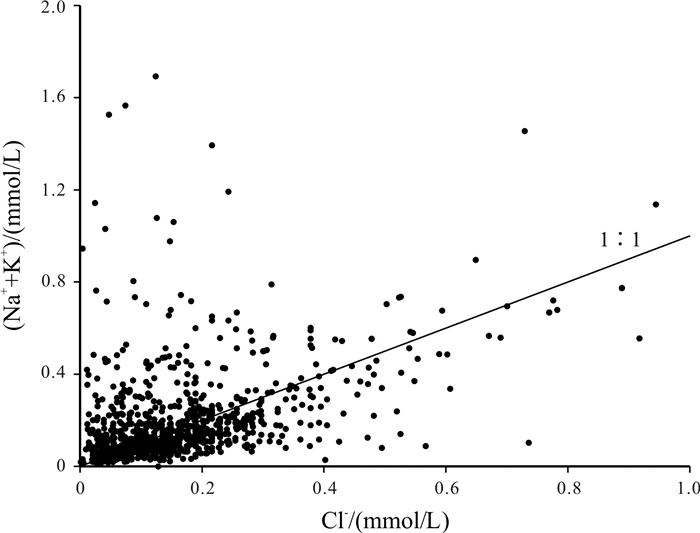
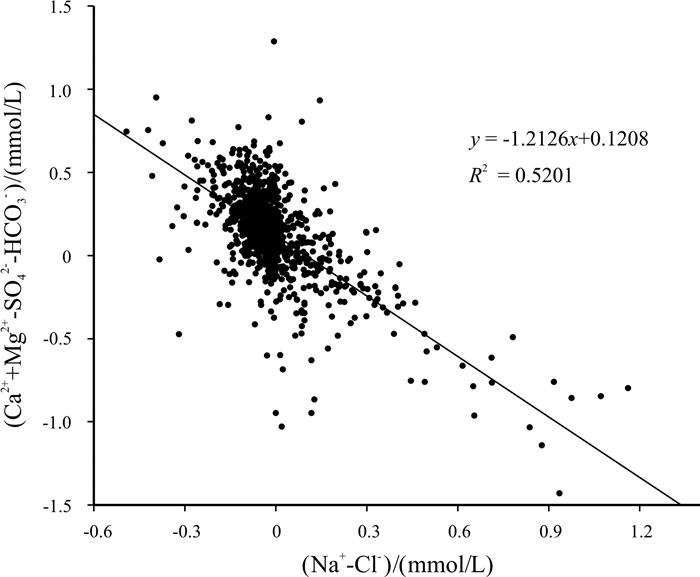
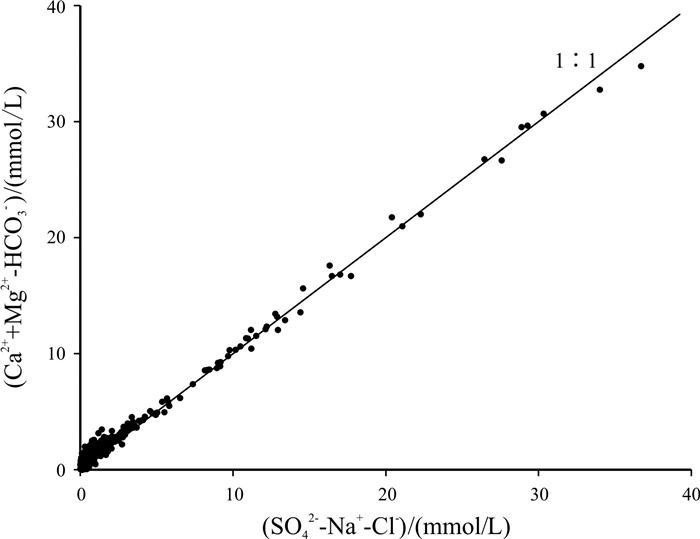
Abstract:Based on the water quality test data of 1 067 boreholes from the water-searching drilling project in Guizhou Province, the study uses the methods including descriptive statistical analysis, variation coefficient, Piper diagram, Schukalev classification, Gibbs diagram and ion ratio coefficient to analyze the hydrochemistry and distribution characteristics of karst water in the study area, and discusses the sources and formation of major ion compositions. The results show that the cations in the groundwater of study area are mainly Ca2+ and Mg2+, and the anions mainly HCO3-. The hydrochemical type of the groundwater is mainly of HCO3-Ca·Mg. The chemical compositions of the groundwater are mainly derived from the lixiviation of carbonate rocks, with Ca2+ and Mg2+ mainly from the dissolution of carbonate rock minerals, which constrain the content of Ca2+ and Mg2+. The main source of Na+ and K+ is affected by the lixiviation of rock salt, as well as other Na- and K-bearing minerals and human activities, while the cation exchange and adsorption has less effect. The anion of SO42- is mainly derived from the leaching of evaporation salts such as gypsum.
-
Key words:
- karst mountainous area /
- groundwater /
- hydrochemical characteristics /
- lixiviation /
- Guizhou Province
-

-
表 1 贵州省主要碳酸盐岩地层2007—2012年钻孔水化学类型统计表
Table 1. Hydrochemical type statistics of main carbonate rock strata based on the water-searching drilling project in Guizhou Province during 2007-2012
界 系 统 组 含水介质类型 钻孔数/个 水化学类型 HCO3-Ca·Mg HCO3-Ca HCO3·SO4-Ca·Mg HCO3·SO4-Ca SO4-Ca·Mg 中生界 三叠系 中统 杨柳井组 白云岩、灰岩 16 14 1 1 关岭组 较纯碳酸盐岩 213 134 14 43 11 11 下统 嘉陵江组 纯灰岩 127 70 23 20 11 3 安顺组 纯白云岩 27 18 6 1 2 大冶组 纯灰岩 9 3 4 2 夜郎组玉龙山段 纯灰岩 24 5 13 1 5 古生界 二叠系 中统 栖霞组-茅口组 纯灰岩 45 9 21 5 10 石炭系 上统 黄龙组-马坪组 纯灰岩 17 3 14 下统 摆佐组 白云岩、灰岩 11 5 2 3 1 泥盆系 上统 高坡场组 纯白云岩 9 8 1 尧梭组 较纯碳酸盐岩 10 6 1 2 1 望城坡组 较纯碳酸盐岩 26 18 2 6 奥陶系 下统 桐梓组-红花园组 白云岩、灰岩 15 10 3 2 寒武系 上统 毛田组 白云岩、灰岩 2 2 追屯组 白云岩、灰岩 9 9 比条组 白云岩、灰岩 5 5 娄山关组 纯白云岩 385 361 7 14 1 2 中统 敖溪组 白云岩、灰岩 4 4 平井组 白云岩、灰岩 12 11 1 花桥组 白云岩、灰岩 2 2 石冷水组 较纯碳酸盐岩 7 7 高台组 不纯碳酸盐岩 64 57 1 3 3 下统 清虚洞组 白云岩、灰岩 23 19 4 新元古界 震旦系 上统 灯影组 纯白云岩 5 3 2 合计 1067 783 115 103 48 18 表 2 研究区地下水主要离子成分特征值统计表
Table 2. Eigenvalues statistics of major ion compositions in groundwater of the study area
指标 单位 最大值 最小值 均值 标准差 变异系数/% pH 8.73 6.45 7.38 0.28 3.8 TDS mg/L 2213.3 138.1 371.2 187.6 50.58 总硬度 mg/L 1866.5 38.4 333.4 167.2 50.18 K+ mg/L 16.3 * 1.3 1.4 107.7 % 14.1 * 1.1 Na+ mg/L 65.4 0.14 3.7 4.7 127.0 % 30.0 * 3.2 Ca2+ mg/L 523.1 10.1 80.9 46.0 56.9 % 95.9 33.5 68.0 Mg2+ mg/L 170.9 1.0 31.8 16.2 50.9 % 56.6 1.0 27.7 HCO3- mg/L 582.4 35.5 312 66.5 21.3 % 99.5 8.0 85.2 SO42- mg/L 1781.8 1.0 68.4 161.6 236.3 % 99.3 * 13.2 Cl- mg/L 33.7 * 5.8 4.8 82.8 % 8.0 * 1.5 * 表示离子浓度小于0.1 mg/L或百分含量小于1%. 百分数值为各离子占阳/阴离子总量百分数. 表 3 不同含水介质中地下水的Ca2+/Mg2+均值
Table 3. Average Ca2+/Mg2+ values of groundwater in different aquifer media
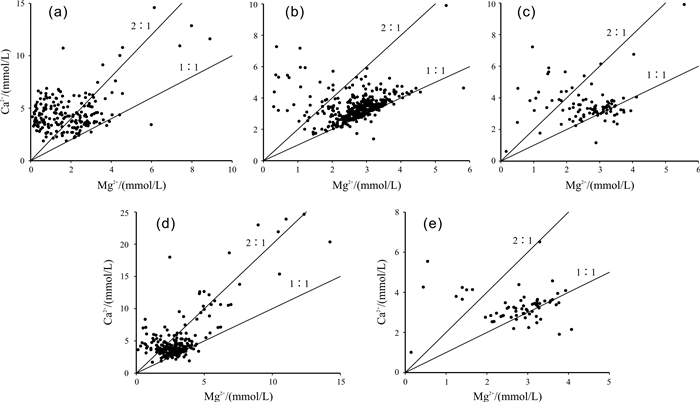
含水介质 纯碳酸盐岩 较纯碳酸盐 不纯碳酸盐岩 纯灰岩 白云岩、灰岩 纯白云岩 均值 Ca2+/Mg2+均值 4.8 1.61 1.47 2.44 2.04 1.45 -
[1] 李玲, 周金龙, 齐万秋, 等. 新疆和田河流域绿洲区浅层地下水水化学特征及成因分析[J]. 水资源与水工程学报, 2018, 29(3): 14-20.
Li L, Zhou J L, Qi W Q, et al. Hydrochemical characteristics and formation reasons of shallow groundwater in oasis area of Hotan River Basin, Xinjiang[J]. Journal of Water Resources and Water Engineering, 2018, 29(3): 14-20.
[2] 栾风娇, 周金龙, 贾瑞亮, 等. 新疆巴里坤-伊吾盆地地下水水化学特征及成因[J]. 环境化学, 2017, 36(2): 380-389.
Luan F J, Zhou J L, Jia R L, et al. Hydrochemical characteristics and formation mechanism of groundwater in plain areas of Barkol-Yiwu Basin, Xinjiang[J]. Environmental Chemistry, 2017, 36(2): 380-389.
[3] 黄科云, 刘德深, 马祖陆, 等. 云南鹤庆西山岩溶地下水主要离子雨季和旱季对比及来源分析[J]. 地球与环境, 2015, 43(2): 183-189.
Huang K Y, Liu D S, Ma Z L, et al. Major ion chemistry and their sources of karstic ground water from the Heqing West Mountain, China during flood and dry seasons[J]. Earth and Environment, 2015, 43(2): 183-189.
[4] 王晓曦, 王文科, 王周锋, 等. 滦河下游河水及沿岸地下水水化学特征及其形成作用[J]. 水文地质工程地质, 2014, 41(1): 25-33, 73.
Wang X X, Wang W K, Wang Z F, et al. Hydrochemical characteristics and formation mechanism of river water and groundwater along the downstream Luanhe River, northeastern China[J]. Hydrogeology & Engineering Geology, 2014, 41(1): 25-33, 73.
[5] 鲁孟胜, 韩宝平, 武凡, 等. 鲁西南地区高氟地下水特征及成因探讨[J]. 中国地质, 2014, 41(1): 294-302. doi: 10.3969/j.issn.1000-3657.2014.01.024
Lu M S, Han B P, Wu F, et al. Characteristics and genesis of high-fluorine groundwater in southwestern Shandong Province[J]. Geology in China, 2014, 41(1): 294-302. doi: 10.3969/j.issn.1000-3657.2014.01.024
[6] 刘影, 王中美, 杨秀丽, 等. 贵安新区东部岩溶地下水水化学特征[J]. 长江科学院院报, 2022, 39(1): 39-46.
Liu Y, Wang Z M, Yang X L, et al. Hydrochemical characteristics of karst groundwater in the east of Gui'an, Guiyang[J]. Journal of Yangtze River Scientific Research Institute, 2022, 39(1): 39-46.
[7] 贺亮亮, 吕广罗, 胡安焱, 等. 基于水化学特征分析的矿井突水水源判别[J]. 中国煤炭地质, 2022, 34(6): 34-39. doi: 10.3969/j.issn.1674-1803.2022.06.07
He L L, Lyu G L, Hu A Y, et al. Mine water bursting water source discrimination based on hydrochemical features analysis[J]. Coal Geology of China, 2022, 34(6): 34-39. doi: 10.3969/j.issn.1674-1803.2022.06.07
[8] 孙岐发, 贾林刚, 田辉, 等. 长春莲花山地区地下水化学特征及成因分析[J]. 地质与资源, 2020, 29(5): 476-482. doi: 10.13686/j.cnki.dzyzy.2020.05.010
Sun Q F, Jia L G, Tian H, et al. Chemical characteristics and genesis analysis of the groundwater in Lianhuashan area, Changchun City[J]. Geology and Resources, 2020, 29(5): 476-482. doi: 10.13686/j.cnki.dzyzy.2020.05.010
[9] 孙厚云, 毛启贵, 卫晓锋, 等. 哈密盆地地下水系统水化学特征及形成演化[J]. 中国地质, 2018, 45(6): 1128-1141.
Sun H Y, Mao Q G, Wei X F, et al. Hydrogeochemical characteristics and formation evolutionary mechanism of the groundwater system in the Hami Basin[J]. Geology in China, 2018, 45(6): 1128-1141.
[10] 李潇瀚, 张翼龙, 王瑞, 等. 呼和浩特盆地地下水化学特征及成因[J]. 南水北调与水利科技, 2018, 16(4): 136-145.
Li X H, Zhang Y L, Wang R, et al. Hydrochemical characteristics and formation mechanism of groundwater in Hohhot Basin[J]. South-to-North Water Transfers and Water Science & Technology, 2018, 16(4): 136-145.
[11] 陈雯, 黎清华, 刘怀庆, 等. 广西钦州港地区地下水水化学特征及形成作用[J]. 华南地质与矿产, 2016, 32(1): 78-84. doi: 10.3969/j.issn.1007-3701.2016.01.010
Chen W, Li Q H, Liu H Q, et al. Analysis on hydrochemical characteristics and formation mechanism of groundwater in Qinzhou Port area, Guangxi, China[J]. Geology and Mineral Resources of South China, 2016, 32(1): 78-84. doi: 10.3969/j.issn.1007-3701.2016.01.010
[12] 张人权, 梁杏, 靳孟贵, 等. 水文地质学基础[M]. 7版. 北京: 地质出版社, 2018.
Zhang R Q, Liang X, Jin M G, et al. Fundamentals of hydrogeology[M]. 7th ed. Beijing: Geological Publishing House, 2018.
[13] 寇文杰. 基于EXCEL的地下水化学舒卡列夫分类方法[J]. 工程勘察, 2013, 41(5): 48-50, 96.
Kou W J. Groundwater chemical Shoka Lev classification method based on EXCEL[J]. Geotechnical Investigation & Surveying, 2013, 41(5): 48-50, 96.
[14] Piper A M. A graphic procedure in the geochemical interpretation of water-analyses[J]. Eos, Transactions, American Geophysical Union, 1944, 25(6): 914-928. doi: 10.1029/TR025i006p00914
[15] Gibbs R J. Mechanisms controlling world water chemistry[J]. Science, 1970, 170(3962): 1088-1090. doi: 10.1126/science.170.3962.1088
[16] 孔令健, 王振龙, 王兵. 阜阳市集中式深层地下水饮用水源地水化学特征及成因分析[J]. 中国农村水利水电, 2020(3): 78-82.
Kong L J, Wang Z L, Wang B. An analysis of the hydro-chemical characteristics and causes of drinking water source of concentrated deep groundwater in Fuyang City[J]. China Rural Water and Hydropower, 2020(3): 78-82.
[17] 唐金平, 张强, 胡漾, 等. 湔江冲洪积扇地下水化学特征及控制因素分析[J]. 环境科学, 2019, 40(7): 3089-3098.
Tang J P, Zhang Q, Hu Y, et al. Groundwater chemical characteristics and analysis of their controlling factors in an alluvial fan of Jianjiang River[J]. Environmental Science, 2019, 40(7): 3089-3098.
[18] 魏兴, 周金龙, 乃尉华, 等. 新疆喀什三角洲地下水化学特征及演化规律[J]. 环境科学, 2019, 40(9): 4042-4051.
Wei X, Zhou J L, Nai W H, et al. Hydrochemical characteristics and evolution of groundwater in the Kashgar delta area in Xinjiang[J]. Environmental Science, 2019, 40(9): 4042-4051.
[19] 刘春燕, 黄冠星, 荆继红, 等. 黄淮海平原地下水化学演化特征、形成机制及其开发利用建议[J]. 中国地质, 2023, 50(6): 1705-1719.
Liu C Y, Huang G X, Jing J H, et al. Characteristics and driving mechanisms of evolution of groundwater chemistry in Huang-Huai-Hai Plain and its exploitation and utilization suggestions[J]. Geology in China, 2023, 50(6): 1705-1719.
[20] Pazand K, Khosravi D, Ghaderi M R, et al. Identification of the hydrogeochemical processes and assessment of groundwater in a semi-arid region using major ion chemistry: A case study of Ardestan Basin in central Iran[J]. Groundwater for Sustainable Development, 2018, 6: 245-254.
[21] Sajil Kumar P J, James E J. Identification of hydrogeochemical processes in the Coimbatore District, Tamil Nadu, India[J]. Hydrological Sciences Journal, 2016, 61(4): 719-731.
[22] Yang Q C, Li Z J, Ma H Y, et al. Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the southeastern part of Ordos Basin, China[J]. Environmental Pollution, 2016, 218: 879-888.
[23] Reddy A G S, Kumar K N. Identification of the hydrogeochemical processes in groundwater using major ion chemistry: A case study of Penna-Chitravathi river basins in southern India[J]. Environmental Monitoring and Assessment, 2010, 170(1/4): 365-382.
[24] Abdelkader R, Larbi D, Rihab H, et al. Geochemical characterization of groundwater from shallow aquifer surrounding Fetzara Lake N. E. Algeria[J]. Arabian Journal of Geosciences, 2012, 5(1): 1-13.
[25] Jankowski J, Acworth R I. Impact of debris-flow deposits on hydrogeochemical processes and the development of dryland salinity in the Yass river catchment, New South Wales, Australia[J]. Hydrogeology Journal, 1997, 5(4): 71-88.
-




 下载:
下载: