Research on the Solid-liquid Interfacial Structure and Properties of Sulfide Minerals and Its Effect on the Flotation Reagent Adsorption
-
摘要:
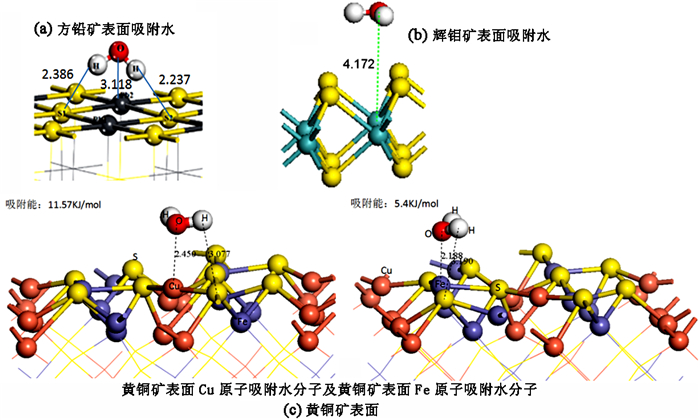
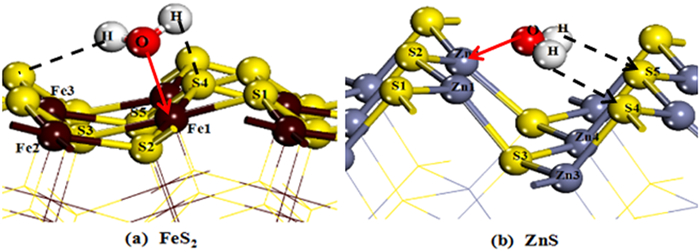
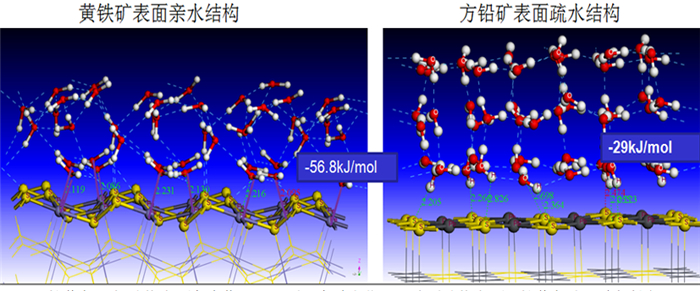
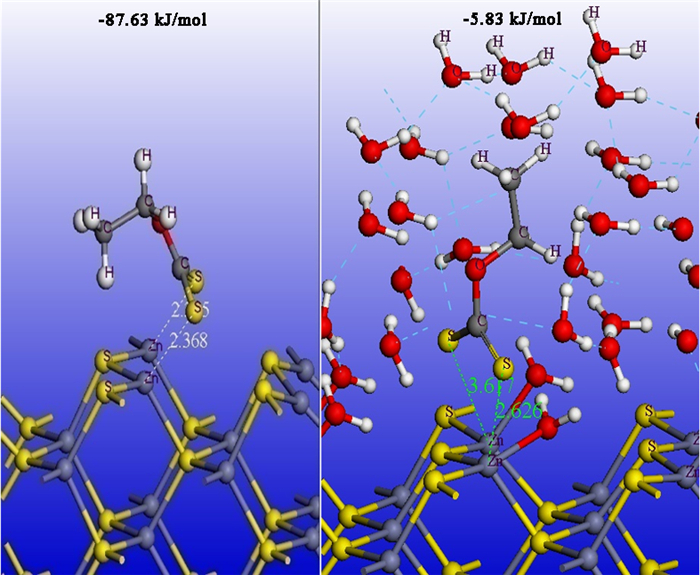
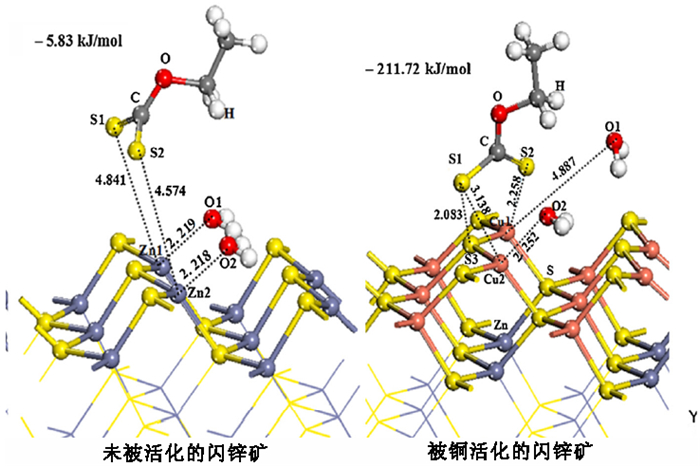
采用密度泛函量子理论研究了硫化矿物表面亲水/疏水结构,揭示了硫化矿物固液界面结构和水化层的性质,采用微量热仪研究了硫化矿物表面润湿热力学和动力学行为,考察了矿物表面亲水和疏水作用对浮选药剂作用的影响。研究结果表明:疏水硫化矿物表面以氢键和范德华力作用为主,亲水硫化矿表面以弱化学吸附键作用为主,固液界面体系下药剂在矿物表面的作用具有水化势垒和竞争吸附效应。根据固液界面作用模型,开发出铜、铅和锌浮选新技术,实现了有色金属硫化矿资源的高效利用。
Abstract:The density functional theory was used to study the hydrophilic and hydrophobic structures on the surface of sulfide minerals. The solid-liquid interface structure and hydration layer properties of sulfide minerals were revealed. The wetting thermodynamic and kinetic behaviors of sulfide minerals were studied by using a microcalorimeter. The effects of hydrophilic and hydrophobic surfaces on the action of flotation agents were investigated. The results show that the surface of hydrophobic sulfide minerals is dominated by hydrogen bonds and Van der Waals forces, and the surface of hydrophilic sulfide minerals is dominated by weak chemical interactions. In the solid-liquid interface system, the adsorption of reagents on mineral surface has a hydration barrier and competitive adsorption effect. Based on the solid-liquid interface model, a new flotation technology of copper, lead and zinc have been developed to achieve clean and highly efficient recycling of non-ferrous metal sulfur resources.
-
Key words:
- sulphide minerals /
- solid-liquid interface /
- hydration layer /
- flotation separation
-

-
表 1 优化的收敛精度
Table 1. Optimized convergence accuracy
矿物类型 黄铁矿 闪锌矿 方铅矿 辉钼矿 黄铜矿 能量收敛精度/(eV·atom-1) 2.0×10-5 2.0×10-5 2.0×10-5 1.0×10-5 2.0×10-5 原子位移收敛精度/Å 0.002 0.002 0.002 0.001 0.002 原子间作用力收敛精度/(eV·Å-1) 0.08 0.05 0.05 0.05 0.05 晶体内应力收敛精度/GPa 0.1 0.1 0.1 0.05 0.1 自洽迭代收敛精度/(eV·atom-1) 2.0×10-6 1.0×10-6 2.0×10-6 1.0×10-6 2.0×10-6 表 2 黄铁矿、方铅矿和闪锌矿比表面积和润湿热(负号表示放热)
Table 2. Specific surface area and wetting heat (negative sign indicates exothermic)of pyrite, galena and sphalerite
矿物 比表面积/(m2·g-1) 润湿热ΔH/(J·m-2) 黄铁矿 0.210 -98.601 闪锌矿 0.115 -4.040 方铅矿 0.228 -2.004 黄铜矿 0.176 -1.892 辉钼矿 0.648 -0.928 表 3 硫化矿物表面水化动力学参数
Table 3. Hydration kinetic parameters of sulfide mineral surface
硫化矿物 速率常数k(×10-3)/s 反应级数n 辉钼矿 26.679 1.179 黄铜矿 18.56 1.363 方铅矿 17.762 1.199 黄铁矿 2.020 0.492 表 4 常见矿物浮选捕收剂基团电负性值[14]
Table 4. Functional electro-negativity of common collectors
硫化矿捕收剂 氧化矿捕收剂 基团 结构 电负性 基团 结构 电负性 硫醇基 R-SH 2.6 羧酸基 R-COOH 4.1 一硫代碳酸基 R-OCOSH 2.8 磺酸基 R-SO2OH 4.3 二硫代碳酸基 R-OCSSH 2.7 羟肟酸基 R-CONOH 3.8 三硫代碳酸基 R-SCSSH 2.6 磷酸基 R-PO(OH)2 4.3 一硫代磷酸基 (RO)2-POSH 3.2 羟基 R-OH 3.9 二硫代磷酸基 (RO)2-PSSH 3.0 伯胺基 R-NH2 3.7 氨基二硫代甲酸基 R2-NCSSH 2.6 二烷基磷酸基 (RO)2-POOH 3.6 黄原酸酯基 R1-OCSS-R2 2.9 氨基硫逐甲酸基 R1-NHCSO-R2 2.8 表 5 陕西某矿不添加石灰铜硫浮选生产指标
Table 5. Industrial Cu-S flotation index without lime
石灰用量
/(kg·t-1)处理量
/(t·d-1)年生产时间/d 原矿Cu品位/% 精矿Cu品位/% 回收率
/%年产铜金属量/t 年产铜精矿/t 不添加 325 350 1.17 15.94 87.45 1 163.85 7 301.44 3.0 325 350 1.18 12.77 80.98 1 086.95 8 511.75 注:捕收剂Z200用量为50 g/t, 无石灰工艺添加抑制剂D82用量350 g/t。 表 6 高碳铅锑矿自然pH铅锑浮选生产指标
Table 6. Industrial Pb-Sb flotation index at natural pH
工艺 产品名称 品位/% 回收率/% 药剂制度 Pb Sb Ag Zn Pb Sb Ag 自然pH界面调控技术 铅锑精矿 31.25 14.55 2 986 5.21 90.86 82.05 83.32 3418A+黑药:400 g/t
高效调整剂:500 g/t铅锑尾矿 0.10 0.10 20 - 9.14 17.95 16.68 原矿 1.06 0.54 116 4.05 100.00 100.00 100.00 传统碱性工艺 铅锑精矿 23.41 12.33 2 179 10.34 86.27 63.29 75.62 黑药:500 g/t
石灰:2.0 kg/t
ZnSO4:800 g/t铅锑尾矿 0.15 0.20 30 13.73 36.71 24.38 原矿 1.05 0.53 118 4.06 100.00 100.00 100.00 注:Ag品位为g/t。 表 7 云南某铅锌矿闪锌矿浮选界面调控技术
Table 7. Flotation interface regulation technology of sphalerite for the lead-zinc ore from Yunnan province
工艺 产品名称 产率
/%锌品位
/%锌回收率/% 药剂制度 传统工艺 锌精矿 30.05 56.45 97.82 硫酸铜:1 000 g/t
丁基黄药:400 g/t尾矿 69.95 0.54 2.18 给矿 100.00 17.34 100.00 新工艺 锌精矿 29.76 57.34 97.79 有机活化剂:600 g/t
硫酸铜:400 g/t
新捕收剂:200 g/t尾矿 70.24 0.55 2.21 给矿 100.00 17.45 100.00 -
[1] 霍夫曼R.固体与表面[M].北京:化学工业出版社, 1996.
[2] Chen Jianhua, Long Xianhao, Zhao Cuihua, et al. DFT calculation on relaxation and electronic structure of sulfide minerals surfaces in presence of H2O molecule[J]. Journal of Central South University, 2014, 21(10):3945-3954. doi: 10.1007/s11771-014-2382-9
[3] 格列姆博茨基BA.浮选过程物理化学基础[M].北京:冶金工业出版社, 1985.
[4] 李少章, 朱书全.对煤粒表面模型的研究[J].煤炭学报, 2004, 29:125-129. http://www.cqvip.com/QK/90748X/200101/4942528.html
[5] 切尔里赫СИ.超声波、磁场和电场对金矿浮选影响的研究[J].国外金属矿选矿, 2004(1):4-7. http://www.cnki.com.cn/Article/CJFDTOTAL-BFHJ201207015.htm
[6] Классен вн. Магнитная обработка воды иводых систем при флотации исяушении руд и углей-в кн[C]. ⅧМеждународный конгресс по обогашенню полезных ископаемых. Т. 1. Л., 1969: 432-442.
[7] Guilherme Ferreira de Lima, Cláudio de Oliveira, HeitorAvelino de Abreu, et al. Water adsorption on the reconstructed(001) chalcopyrite surfaces[J]. The Journal of Physical and Chemistry, 2011, 115(21):10709-10717. http://www.mendeley.com/catalog/water-adsorption-reconstructed-001-chalcopyrite-surfaces/
[8] Cuihua Zhao, Jianhua Chen, Bozeng Wu, et al. Density functional theory study on natural hydrophobicity of sulfide surfaces[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2):491-498. doi: 10.1016/S1003-6326(14)63087-9
[9] Jianhua Chen, Xianhao Long, Ye Chen. Comparison of multilayer water adsorption on the hydrophobic galena (PbS) and hydrophilic pyrite (FeS2) surfaces:A DFT study[J]. The Journal of Physical and Chemistry, 2014, 118(22):11657-11665. http://www.mendeley.com/research/comparison-multilayer-water-adsorption-hydrophobic-galena-pbs-hydrophilic-pyrite-fes2-surfaces-dft-s/
[10] Cuihua Zhao, Jianhua Chen, Xianhao Long, et al. Study of H2O adsorption on sulfides surfaces and thermokineticanalysis[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(2):605-609. doi: 10.1016/j.jiec.2013.05.021
[11] Long Xianhao, Chen Jianhua, Chen Ye. Adsorption of ethylxanthate on ZnS(110) surface in the presence of water molecules:A DFT study[J]. Applied Surface Science, 2016, 370:11-18. doi: 10.1016/j.apsusc.2016.02.094
[12] 陈建华, 冯其明, 卢毅屏.浮选药剂的亲固能计算[J].中国有色金属学报, 1999, 9(20):351-357. http://www.cqvip.com/QK/97361X/1999002/3535055.html
[13] 王文强, 何利华, 赵中伟.钨钼分离吸附剂的选择性判据[J].中国有色金属学报, 2015, 25(8):2236-2242. http://www.cqvip.com/QK/97361X/201508/665861836.html
[14] 王淀佐.浮选剂作用原理及应用[M].北京:冶金工业出版社, 1982.
-



 下载:
下载: