Experimental Study on Flotation XinJiang Bertrandite Using Combination Collectors
-
摘要:
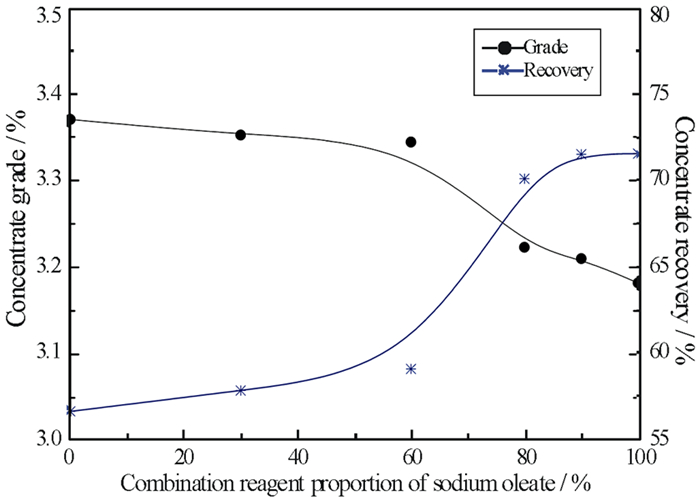
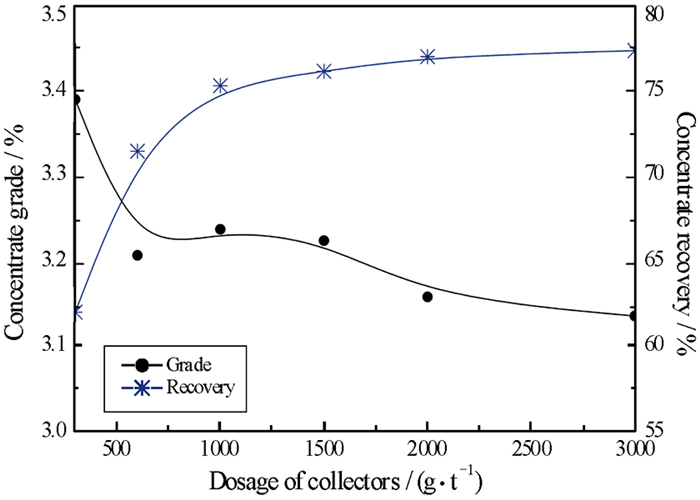
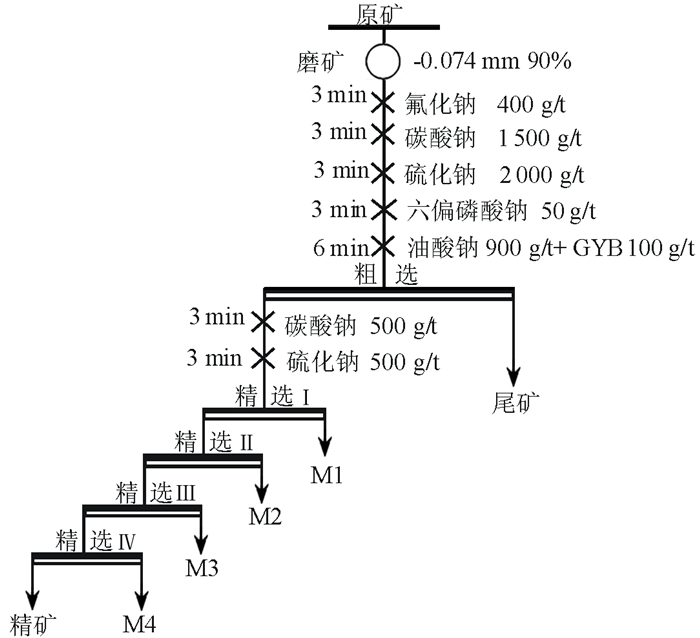
针对新疆某含铍(BeO)0.45%的羟硅铍石浮选中,单一捕收剂选择性较低、精矿品位不高的问题,试验采用油酸钠和苯甲羟肟酸组合捕收剂。通过浮选条件试验的优化,结果发现,当组合捕收剂用量为1 000 g/t时(油酸钠GA6FA苯甲羟肟酸质量比为9 GA6FA 1),二者预先混合后加入,浮选效果最佳,经过四次开路精选,能够得到BeO品位10.22%的铍精矿,富集效果较好,可以为类似的羟硅铍石资源回收提供有效的技术支撑。混合药剂的溶液化学分析表明,在二者混合的水溶液pH值8.25左右时,苯甲羟肟酸分子组分和离子组分浓度相当,这可能是产生预先混合协同效果的原因。
Abstract:In the flotation of bertrsandite in Xinjiang with the grade of BeO 0.45%, the selectivity was a bit low and the grade of concentration is not quite high when single collector was used. In order to deal with the problem above, sodium oleate and benzohydroxamic acid were used together. It is found after optimizing the flotation condition tests that when the proportion of sodium oleate and benzohydroxamic acid was 9 GA6FA 1, they are mixed before adding to the pulp and in this condition the optimum flotation effect can be gotten. When the dosage of collector was 1 000 g/t and collector was added beforehand, beryllium concentrate with grade 10.22% could be received after four times open circuit concentration, which showed the better concentration effect and provided effective technological support for recovering similar bertrsandite. The solution chemistry results of mixed reagents showed that when the pH value of mixed solution was approximately 8.25, the molecular concentration of benzohydroxamic acid is quite similar to ion concentration, which may results in assembly synergy.
-
Key words:
- collector /
- flotation /
- combination /
- bertrsandite /
- sodium oleate /
- benzohydroxamic acid
-

-
表 1 矿石化学多元素分析结果 /%
Table 1. The results of multielement analysis of raw ore
成分 BeO SiO2 Al2O3 CaO MgO K2O Na2O F TFe 含量 0.45 66.48 14.20 2.47 3.23 3.19 3.54 1.00 2.99 表 2 捕收剂加入方式试验结果
Table 2. Results of the experiments on collector combinations
方案 产品名称 产率/% BeO品位/% BeO回收率/% (1) 精矿 9.93 3.210 71.51 (2) 精矿 10.06 3.206 71.63 (3) 精矿 9.95 3.220 71.47 (4) 精矿 10.37 3.233 73.05 表 3 精选开路试验结果
Table 3. Results of the open concentration flotation
产品名称 产率/% BeO品位/% BeO回收率/% 精矿 1.54 10.22 34.81 M1 3.84 1.134 9.63 M2 2.68 2.227 13.20 M3 1.88 2.734 11.37 M4 0.43 4.432 4.22 尾矿 89.63 0.135 26.77 原矿 100.00 0.45 100.00 -
[1] 李振军.金属铍冶炼进展[J].中国有色冶金, 2006(6):18-23. doi: 10.3969/j.issn.1672-6103.2006.06.005
[2] 李爱民, 蒋进光, 王晖, 等.含铍矿物浮选研究现状与展望[J].稀有金属与硬质合金, 2008, 36(3):58-61. doi: 10.3969/j.issn.1004-0536.2008.03.015
[3] 张玲, 林德松.我国稀有金属资源现状分析[J].地质与勘探, 2004, 40(1):26-30. doi: 10.3969/j.issn.0495-5331.2004.01.006
[4] 郭景阳.铍铜工业近况[J].上海金属:有色分册, 1989(3):47-52. http://d.old.wanfangdata.com.cn/NSTLQK/10.1111-j.1399-6576.2010.02234.x/
[5] 成泉辉.非绿柱石铍矿生产工业氧化铍的工艺研究与实践[J].中国有色冶金, 2006(6):24-27. doi: 10.3969/j.issn.1672-6103.2006.06.006
[6] 张志敏, 晏惕非, 尹江生.绿柱石浮选新工艺[J].矿产综合利用, 1988(4):29-31. http://cdmd.cnki.com.cn/Article/CDMD-10533-2006036713.htm
[7] 何炯奎.绿柱石的工业浮选实践及其控制[J].稀有金属, 1981(5):24-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS198105004.htm
[8] 何建璋.可可托海三号脉铍矿石的综合利用[J].新疆有色金属, 2003, 26(4):22-24. http://d.old.wanfangdata.com.cn/Periodical/xjysjs200304006
[9] 王毓华, 于福顺, 陈兴华, 等.锂辉石与绿柱石浮选分离的试验研究[J].稀有金属, 2005, 29(3):320-324. doi: 10.3969/j.issn.0258-7076.2005.03.015
[10] 赵笑益, 齐建云.某羟硅铍石矿石选冶试验研究[J].现代矿业, 2015(10):85-88. doi: 10.3969/j.issn.1674-6082.2015.10.027
[11] 郑元泽, 王国全.新疆杨庄羟硅铍石的浮选试验研究[J].新疆有色金属, 2012, 35(1):66-68. http://d.old.wanfangdata.com.cn/Periodical/xjysjs201201022
[12] 张霄.新疆铍矿资源找矿类型浅议[J].新疆有色金属, 2016, 39(4):19-22. http://d.old.wanfangdata.com.cn/Periodical/xjysjs201604007
[13] 韩兆元, 高玉德, 王国生, 等.组合捕收剂对黑钨矿疏水行为的影响研究[J].稀有金属, 2012(6):973-978. http://d.old.wanfangdata.com.cn/Conference/7539831
[14] 刘晓文, 毛小西, 刘庄, 等.羟硅铍石型铍矿的工艺矿物学研究[J].矿物学报, 2010(S1):61-63. http://d.old.wanfangdata.com.cn/Conference/7339972
[15] 胡岳华, 孙伟, 黄红军, 等.一种羟硅铍石类铍矿浮选捕收剂及其应用: CN101716559A[P].2010-06-02.
[16] 王军, 程宏伟, 赵红波, 等.油酸钠作用下金红石的浮选行为及作用机理[J].中国有色金属学报, 2014, 24(3):820-825. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb201403033
-




 下载:
下载: