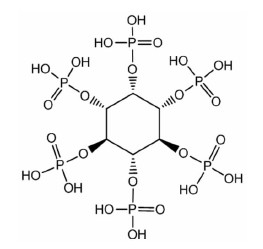
Synthesis and Application of Organophosphorus Reagents For Mineral Processing and Metallurgy
-
摘要:
有机磷选冶药剂具有灵活多变的极性基和非极性基,使其具有可调可控的性能与广阔的应用前景。介绍了磷酸酯、烃基膦酸、烃基膦酸酯、烃基亚膦酸、二烃基次膦酸、二烃基次膦酸酯、三烃基氧化膦、二烃基二硫代磷酸、二烃基硫代次膦酸及其衍生物等有机磷药剂的结构特点、合成方法以及其在矿物加工、冶金中用作捕收剂、萃取剂、缓蚀剂、抑制剂和浸出剂的研究进展,为有机磷药剂的发展提供了思路。
Abstract:Organophosphorus mineral processing and metallurgical reagents have flexible and variable polar and nonpolar groups, which makes them have adjustable and controllable performance and wide application prospects. The structural characteristics and synthesis methods of organophosphorus reagents, including alkylphosphate, alkylphosphonic acid, alkylphosphonate, alkylphosphonous acid, dialkylphosphinic acid, dialkylphosphinate, trialkylphosphine oxide, dialkyl dithiophosphoric acid, dialkyl thiophosphinic acid and their derivatives, were introduced. The applications of the organophosphorus reagents in the field of mineral processing and metallurgical engineering, including collector, extractant, corrosion inhibitor, depressant and lixiviant, were also discussed. The ideas for the development of organophosphorus reagents were provided.
-
Key words:
- organic phosphorus /
- collector /
- extractant /
- corrosion inhibitor /
- synthesis /
- application
-

-
[1] Pasek M A, Gull M, Herschy B, Phosphorylation on the early earth[J]. Chemical Geology, 2017, 475:149-170. doi: 10.1016/j.chemgeo.2017.11.008
[2] 林强,王淀佐.有机磷浮选剂[J].湖南有色金属,1989,5(1):16-20. http://d.old.wanfangdata.com.cn/Thesis/Y1810136
[3] 师亚宁.十二烷基磷酸单酯的合成与性能研究[D].太原:中国日用化学工业研究院,2018.
[4] 张辉,李效军.脱水磷酰氯法合成磷酸二异辛酯[J].化学试剂,2017,39(9):1003-1006. http://d.old.wanfangdata.com.cn/Periodical/huaxsj201709023
[5] Suresh A, Srinivasan T G, Vasudeva Rao P R. The effect of the structure of trialkyl phosphates on their physicochemical properties and extraction behavior[J]. Solvent Extraction and Ion Exchange, 2009, 27(2):258-294. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/07366290802674481
[6] Maranescu B, Visa A, Ilia G, et al. Synthesis and structural characterization of 2-D layered copper(Ⅱ) styrylphosphonate coordination polymers[J]. Journal of Coordination Chemistry, 2014, 67(9):1562-1572. doi: 10.1080/00958972.2014.928289
[7] Kieczykowski G R, Jobson R B, Melillo D G, et al. Preparation of (4-amino-1-hydroxybutylidene)bisphosphonic acid sodium salt, MK-217(alendronate sodium). An improved procedure for the preparation of 1-hydroxy-1,1-bisphosphonic acids[J]. Journal of Organic Chemistry, 1995, 60(25):8310-8312. doi: 10.1021/jo00130a036
[8] Neu J, Fischer J, Fodor T, et al. Industrial process for the synthesis of 2-substituted 1-(hydroxy-ethylidene)-1,1-bisphosphonic acids of high purity and the salts thereof:US20060122395[P]. 2006-06-08.
[9] 邓晓洋,王微宏,郭丹峰,等.N-(4-甲基苯基)-α-氨基苄基磷酸的合成及其浮选性能[J].应用化工,2012,41(10):1685-1688. http://d.old.wanfangdata.com.cn/Periodical/sxhg201210004
[10] Wu M S, Chen R Y, Huang Y. Convenient synthesis of analogs of aminomethylene gem-diphosphonic acid from amines without catalyst[J]. Synthetic Communications, 2004, 34(8):1393-1398. doi: 10.1081/SCC-120030688
[11] 钟宏,李方旭,王帅,等.一种α-羟基不饱和烷基膦酸及其制备方法与浮选应用:201310571656.4[P].2014-03-05.
[12] Li L Y, Xu S M, Ju Z J, et al. Dialkyl phosphinic acids:Synthesis and applications as extractant for nickel and cobalt separation[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1):205-210.
[13] Huang K H, Jia Y, Wang S, et al. A novel method for the synthesis of styryl phosphonate monoester and its application in La (III) extraction[J]. Journal of Rare Earths, 2020, 38(6):649-656. doi: 10.1016/j.jre.2019.09.003
[14] Verbelen B, Dehaen W, Binnemans K. Selective substitution of POCl3 with organometallic reagents:Synthesis of phosphinates and phosphonates[J]. Synthesis, 2018, 50(10):2019-2026. doi: 10.1055/s-0037-1609435
[15] 王颖.二烷基次膦酸及三烷基氧化磷合成方法的改进[D].湘潭:湘潭大学,2012.
[16] 徐庆华.二异丁基二硫代磷酸钠的合成研究[J].山东化工,2019,48(22):20-21. doi: 10.3969/j.issn.1008-021X.2019.22.008
[17] 于奉先,贾彩,吴王锁,等.二烷基二硫代次膦酸的合成[J].合成化学,2008,16(5):558-560. doi: 10.3969/j.issn.1005-1511.2008.05.016
[18] 孙青,冯其明,石晴.十二烷基磷酸酯钾在菱锌矿表面的吸附机理[J].中南大学学报(自然科学版),2018,49(8):1845-1850. http://d.old.wanfangdata.com.cn/Periodical/zngydxxb201808001
[19] Liu W P, Wang Z X, Wang X M, et al. Smithsonite flotation with lauryl phosphate[J]. Minerals Engineering, 2020, 147:106155. doi: 10.1016/j.mineng.2019.106155
[20] Srinivas K, Sreenivas T, Padmanabhan N P H, et al. Studies on the application of alkyl phosphoric acid ester in the flotation of wolframite[J]. Mineral Processing and Extractive Metallurgy Review, 2004, 25(4):253-267. doi: 10.1080/08827500490472013
[21] Fan H L, Qin J Q, Liu J, et al. Investigation into the flotation of malachite, calcite and quartz with three phosphate surfactants[J]. Journal of Materials Research and Technology, 2019, 8(6):5140-5148. doi: 10.1016/j.jmrt.2019.08.037
[22] Zheng X P, Misra M, Smith R W, et al. Fersmite flotation with diphosphonic acid and other collectors[J]. Minerals Engineering, 1996, 9(3):331-341. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=494f34139b240df6700e9fc3203157e6
[23] 宫贵臣,刘杰,韩跃新,等.苯乙烯膦酸在锡石(100)表面吸附的密度泛函理论研究[J].中南大学学报(自然科学版),2018,49(12):2901-2907. doi: 10.11817/j.issn.1672-7207.2018.12.001
[24] Liu Q, Peng Y J. The development of a composite collector for the flotation of rutile[J]. Minerals Engineering, 1999,12(12):1419-1430. doi: 10.1016/S0892-6875(99)00131-4
[25] Tan W, Liu G Y, Qin J Q, et al. Hemimorphite flotation with 1-hydroxydodecylidene-1,1-diphosphonic acid and its mechanism[J]. Minerals, 2018, 8(2):38. doi: 10.3390/min8020038
[26] Li F X, Zhong H, Xu H F, et al. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite[J]. Minerals Engineering, 2015, 71:188-193. doi: 10.1016/j.mineng.2014.11.013
[27] Huang K H, Huang X P, Jia Y, et al. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation[J]. Minerals Engineering, 2019, 142:105895. doi: 10.1016/j.mineng.2019.105895
[28] Zhong H, Huang Z R, Zhao G, et al. The collecting performance and interaction mechanism of sodium diisobutyl dithiophosphinate in sulfide minerals flotation[J]. Journal of Materials Research and Technology, 2015, 4(2):151-161. doi: 10.1016/j.jmrt.2014.12.003
[29] 沈纬,王英,傅洵.硫酸铝生产过程中的萃取法除铁[J].应用化学,2002,19(5):464-467. doi: 10.3969/j.issn.1000-0518.2002.05.013
[30] Khaironie M T, Masturah M, Meor Yusoff M S, et al. Solvent extraction of light rare earth ions using D2EHPA from nitric acid and sulphuric acid solutions[J]. Advanced Materials Research, 2014, 970:209-213. doi: 10.4028/www.scientific.net/AMR.970.209
[31] Wang J L, Xu S M, Li L Y, et al. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction-separation performance of heavy rare earths from HNO3 solutions[J]. Hydrometallurgy, 2013, 137:108-114. doi: 10.1016/j.hydromet.2013.05.010
[32] Ahmadipour M, Rashchi F, Ghafarizadeh B, et al. Synergistic effect of D2EHPA and Cyanex 272 on separation of zinc and manganese by solvent extraction.[J]. Separation Science and Technology, 2011, 46(15):2305-2312. doi: 10.1080/01496395.2011.594848
[33] Modolo G, Odoj R. Influence of the purity and irradiation stability of Cyanex 301 on the separation of trivalent actinides from lanthanides by solvent extraction[J]. Journal of Radioanalytical and Nuclear Chemistry, 1998, 228(1-2):83-89. doi: 10.1007/BF02387304
[34] Devi N B, Mishra S. Solvent extraction equilibrium study of manganese(II) with Cyanex 302 in kerosene[J]. Hydrometallurgy, 2010, 103(1-4):118-123. doi: 10.1016/j.hydromet.2010.03.007
[35] Chandrasekar A, Sivaraman N, Ghanty T K, et al. Experimental evidence and quantum chemical insights into extraction and third phase aggregation trends in Ce(IV) organophosphates[J]. Separation and Purification Technology, 2019, 217:62-70. doi: 10.1016/j.seppur.2019.02.007
[36] 崔涛,徐庆鑫,袁野,等.采用磷酸三丁酯(TBP)从含锌烟尘氯化浸出液中萃取锌[J].矿冶,2019,28(5):65-68,102. http://d.old.wanfangdata.com.cn/Periodical/ky201905012
[37] 石成龙,宋桂秀,秦亚茹,等.磷酸三丁酯/丁酸乙酯体系协同萃取提锂的研究[J].化学工程,2020,48(2):16-19,73. doi: 10.3969/j.issn.1005-9954.2020.02.004
[38] 李树森,袁承业.烷基膦酸二烷基酯萃取铀、钍反应中取代基效应的分子力学研究[J].原子能科学技术,1989,23(6):38-46. http://www.cqvip.com/qk/90373X/198906/177740.html
[39] Li K, Chen J, Zou D, et al. A novel extractant 2-ethylhexyl bis(2-ethylhexyl)phosphinate for cerium(IV) and fluorine extraction from nitric acid system[J]. Hydrometallurgy, 2019, 186:143-150. doi: 10.1016/j.hydromet.2019.04.014
[40] Iyer J N, Dhadke P M. Liquid-liquid extraction and separation of gallium (Ⅲ), indium (Ⅲ), and thallium (Ⅲ) by Cyanex-925[J]. Separation Science and Technology, 2001, 36(12):2773-2784. doi: 10.1081/SS-100107225
[41] Notoya T, Otieno-Alego V, Schweinsberg D P. The corrosion and polarization behaviour of copper in domestic water in the presence of Ca, Mg and Na-Salts of phytic acid[J]. Corrosion Science, 1995, 37(1):55-65. doi: 10.1016/0010-938X(94)00105-F
[42] 郑细鸣,涂伟萍.膦酸缓蚀剂的合成及其缓蚀性能研究[J].腐蚀与防护,2004,25(10):422-425. doi: 10.3969/j.issn.1005-748X.2004.10.003
[43] Chen W, Feng Q M, Zhang G F, et al. Investigations on flotation separation of scheelite from calcite by using a novel depressant:Sodium phytate[J]. Minerals Engineering, 2018, 126:116-122. doi: 10.1016/j.mineng.2018.06.008
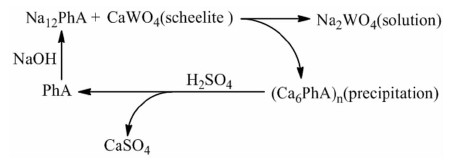
[44] Zhu X R, Liu X H, Zhao Z W, et al. A green method for decomposition of scheelite under normal atmospheric pressure by sodium phytate[J]. Hydrometallurgy, 2020, 191:105234. doi: 10.1016/j.hydromet.2019.105234
-




 下载:
下载: