Effect of Aluminum Impurity on Extraction of Rare Earth From Ammonium Salt Leaching Solution with Phosphate Ester Mixed Extractant
-
摘要:
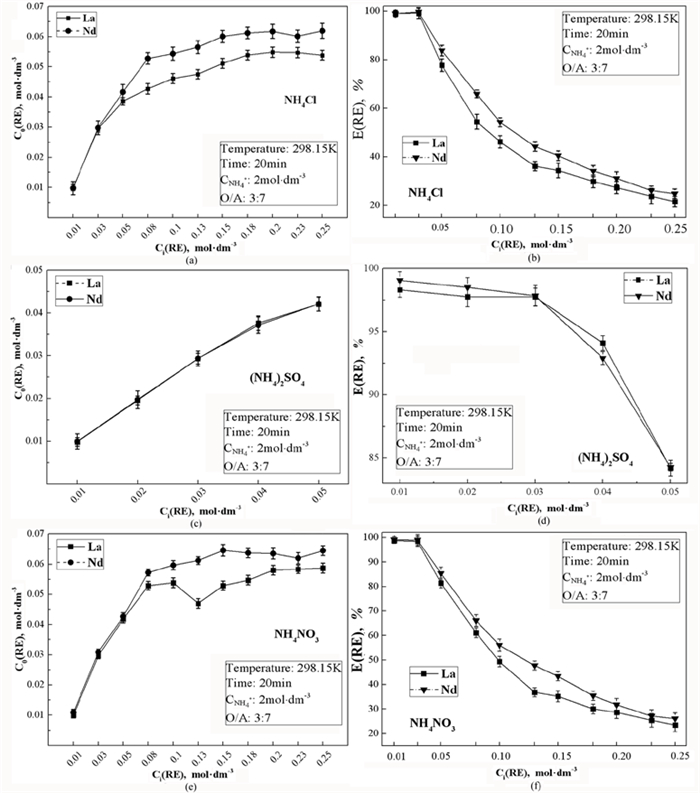
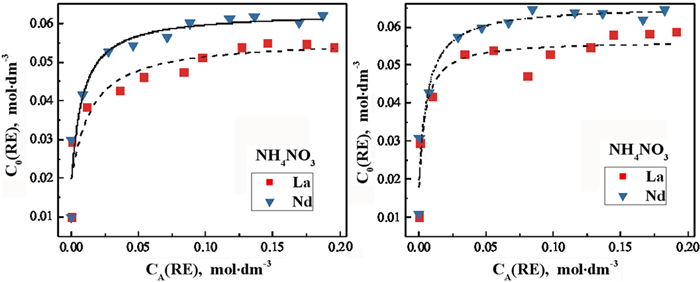
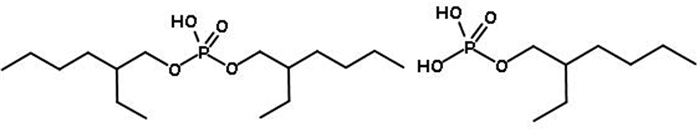
风化壳淋积型稀土矿是我国重要的战略性资源,使用原地浸出开采工艺得到的稀土浸出液中铝含量较高,是主要的杂质离子。为了从该浸出液中一步法分离富集稀土,探讨了一种磷酸酯混合——萃取剂P0261(2-乙基己基磷酸酯,单酯和二酯1 GA6FA 1混合)在NH4Cl、(NH4)2SO4、NH4NO3三种模拟浸出液中对稀土La3+、Nd3+的萃取行为,并分析杂质铝离子对稀土萃取行为的影响。试验结果表明:在三种铵盐溶液中,萃取剂均能够有效萃取分离稀土离子La3+、Nd3+;加入Al3+后,稀土萃取率E会随Al3+浓度增大而减小。但当Al3+浓度在300 mg·dm-3以内时,E降幅缓慢,最大下降值为14.68%。通过分析负载有机相,Al3+比RE3+更易与P=O形成配位键,且铵根离子浓度变化会影响P=O→RE配位键的形成,从而影响萃取行为。因此,将浸出液中的杂质铝离子浓度控制在300 mg·dm-3以内,则可以使用萃取剂P0261一步法萃取分离稀土。
Abstract:The weathered shell leaching type rare earth ore is an important strategic resource in China. The rare earth leaching solution obtained by in situ leaching process has high content of Al3+ and is the main impurity ion. In order to separation rare earth ions from leaching solution by one-step way, the extraction behavior of a phosphate ester extractant, P0261 (2-ethylhexyl phosphate, 1 GA6FA 1 mixture of mono ester and diester), was investigated for rare earth ions La3+、Nd3+ in three simulated leaching solutions of NH4Cl, (NH4)2SO4 and NH4NO3, and the effect of impurity Al3+ on the extraction behavior of rare earth was also analyzed. The results show that the extractant P0261 has good extraction affinity towards rare earth ions La3+ and Nd3+ in the three kinds of ammonium salts solution. With the addition of Al3+, the extraction rate E of rare earth ions decreases with the increase of Al3+ concentration. E declines slowly when C(Al3+) at 0~300 mg·dm-3, while the maximum reduction of E is 14.68%. By analyzed the load organic phase, Al3+ was easier to coordination bonds with P=O than RE3+, and the change of NH4+ concentration has novel effect on the formation of P=O→RE coordination bonds, which was one of the reasons caused the change of rare earth extraction behavior. From experimental results, control the C(Al3+) below 300 mg·dm-3 in in-situ leaching solution, the effect of impurity ion on selective extraction and separation rare earths by one-step way using extractant P0261 is accepted.
-
Key words:
- rare earth /
- Al3+ /
- acidic phosphorus extractor /
- extraction /
- ammonium salt
-

-
表 1 NH4Cl中含P基团峰吸收频率变化
Table 1. Peak absorption frequency changes of phosphorus-containing groups in NH4Cl
体系 编号 C(NH4+)/mol·dm-3 P-O-RE P=O P-O-C 皂后萃取剂 0 2 730.43 2 323.42 1 648.30 1 201.58 1 030.25 Nd3+-NH4Cl 1 0 2 727.84 2 309.77 1 640.60 1 194.40 1 031.74 2 0.5 2 727.74 2 308.64 1 636.49 1 194.12 1 032.23 3 1.0 2 727.49 2 310.30 1 632.76 1 196.83 1 031.64 4 1.5 2 728.06 2 309.43 1 641.21 1 196.96 1 031.99 5 2.0 2 727.58 2 309.47 1 646.94 1 197.82 1 033.9 6 2.5 2 728.19 2 309.68 1 647.75 1 198.34 1 034.81 7 3.0 2 727.53 2 309.18 1 643.07 1 198.73 1 036.07 La3+-NH4Cl 22 0 2 723.16 2 311.75 1 640.02 1 198.76 1 031.54 23 0.5 2 729.38 2 309.82 1 646.68 1 199.67 1 037.88 24 1.0 2 724.73 2 316.57 1 642.20 1 199.73 1 031.59 25 1.5 2 724.92 2 312.23 1 647.24 1 199.94 1 034.38 26 2.0 2 723.59 2 312.71 1 641.64 1 201.10 1 036.48 27 2.5 2 723.66 2 308.86 1 640.91 1 201.67 1 036.82 28 3.0 2 726.22 2 310.79 1 642.51 1 201.16 1 036.80 表 2 (NH4)2SO4中含磷基团峰吸收频率变化
Table 2. Peak absorption frequency changes of phosphorus-containing groups in (NH4)2SO4 solution
体系 编号 C(NH4+)/mol·dm-3 P-O-RE P=O P-O-C 皂后萃取剂 0 2 730.43 2 323.42 1 648.30 1 201.58 1 030.25 Nd3+-(NH4)2SO4 8 0 2 726.13 2 311.63 1 641.74 1 198.00 1 035.48 9 0.5 2 728.06 2 309.61 1 646.95 1 198.12 1 036.82 10 1.0 2 728.83 2 309.68 1 646.75 1 198.61 1 036.23 11 1.5 2 728.70 2 309.87 1 646.88 1 198.67 1 036.11 12 2.0 2 727.38 2 310.02 1 646.69 1 198.68 1 036.31 13 2.5 2 729.52 2 316.56 1 646.69 1 200.46 1 036.04 14 3.0 2 728.34 2 309.85 1 646.66 1 200.91 1 036.59 La3+-(NH4)2SO4 29 0 2 730.30 2 309.58 1 642.83 1 198.86 1 031.19 30 0.5 2 730.01 2 309.43 1 641.34 1 199.39 1 031.37 31 1.0 2 730.21 2 309.92 1 647.04 1 201.86 1 030.82 32 1.5 2 730.76 2 309.92 1 646.17 1 200.22 1 031.15 33 2.0 2 729.49 2 309.88 1 646.50 1 200.98 1 033.06 34 2.5 2 729.77 2 311.20 1 646.84 1 201.08 1 030.20 35 3.0 2 729.52 2 310.33 1 646.52 1 201.95 1 030.35 表 3 NH4NO3中含磷基团峰吸收频率变化
Table 3. Peak absorption frequency changes of phosphorus-containing groups in NH4NO3 solution
体系 编号 C(NH4+)/mol·dm-3 P-O-RE P=O P-O-C 皂后萃取剂 0 2 730.43 2 323.42 1 648.30 1 201.58 1 030.25 Nd3+-NH4NO3 15 0 2 730.86 2 306.93 1 636.49 1 196.66 1 034.55 16 0.5 2 729.71 2 306.93 1 636.09 1 197.58 1 036.39 17 1.0 2 730.62 2 305.91 1 636.34 1 200.01 1 037.18 18 1.5 2 730.59 2 307.41 1 636.28 1 201.14 1 037.12 19 2.0 2 730.27 2 306.45 1 636.54 1 201.67 1 038.13 20 2.5 2 731.28 2 307.41 1 636.88 1 204.79 1 038.34 21 3.0 2 731.18 2 308.38 1 636.50 1 209.20 1 038.52 La3+-NH4NO3 36 0 2 729.10 2 309.61 1 646.55 1 200.01 1 037.14 37 0.5 2 728.10 2 309.82 1 646.51 1 200.78 1 036.86 38 1.0 2 729.09 2 309.86 1 646.61 1 203.30 1 036.87 39 1.5 2 728.77 2 309.65 1 646.52 1 200.05 1 036.57 40 2.0 2 728.02 2 309.93 1 646.54 1 200.47 1 036.67 41 2.5 2 728.53 2 309.54 1 646.58 1 199.94 1 036.66 42 3.0 2 728.64 2 309.89 1 646.51 1 199.30 1 037.38 -
[1] 池汝安, 田君.风化壳淋积型稀土矿化工冶金[M].北京:科学出版社, 2006.
[2] 程建忠, 车丽萍.中国稀土资源开采现状及发展趋势[J].稀土, 2010, 31(2):65-69. http://d.old.wanfangdata.com.cn/Periodical/xitu201002015
[3] 池汝安, 田君.风化壳淋积型稀土矿评述[J].中国稀土学报, 2007, 25(6):641-650. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb200706001
[4] Tian J, Yin J Q, Chen K H, et al. Extraction of rare earths from the leach liquor of the weathered crust elution-de-posited rare earth ore with non-precipitation[J]. International Journal of Mineral Processing, 2011, 98(3-4):125-131.
[5] 田君.风化壳淋积型稀土矿浸取动力学与传质研究[D].中南大学, 2010.
[6] 吴文远, 张丰云, 尹少华, 等.皂化P507体系有机相负载硅对稀土La萃取的影响[J].东北大学学报(自然科学版), 2013, 34(5):679-682. http://d.old.wanfangdata.com.cn/Periodical/dbdxxb201305016
[7] Wu W Y, Zhang F Y, Bian X, et al. Effect of loaded organic phase containing mixtures of silicon and aluminum, single iron on extraction of lanthanum in saponification P507-HCl system[J]. Journal of Rare Earths (English Edition), 2013, 31(7):722-726. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgxtxb-e201307014
[8] 邱廷省, 伍红强, 方夕辉, 等.风化壳淋积型稀土矿提取除杂技术现状及进展[J].稀土, 2012, 33(4):81-85. http://d.old.wanfangdata.com.cn/Periodical/xitu201204017
[9] 池汝安, 王淀佐.某复杂溶液沉淀稀土草酸用量分析及试验研究[J].稀土, 1992(4):11-14.
[10] 池汝安, 李秀芬, 徐景明, 等.碳酸氢铵用于含铝溶液中分离稀土的理论分析研究[J].矿产综合利用, 1993(4):39-42. http://d.old.wanfangdata.com.cn/Conference/285821
[11] 董金诗, 黄小卫, 冯宗玉, 等.离子吸附型稀土矿低浓度浸出液分步萃取富集新技术研究进展[C]//全国稀土化学与冶金学术研讨会暨中国稀土学会稀土化学与湿法冶金、稀土火法冶金专业委员会工作会议论文摘要集, 2014(9): 30-31.
[12] 施展华, 朱健玲, 程哲, 等.离子型稀土开采提取技术的现状与发展[J].世界有色金属, 2018(17):48-50. http://d.old.wanfangdata.com.cn/Periodical/sjysjs201817028
[13] 田君, 尹敬群, 谌开红, 等.风化壳淋积型稀土矿浸出液沉淀浮选溶液化学分析[J].稀土, 2011, 32(4):1-7. http://d.old.wanfangdata.com.cn/Periodical/xitu201104001
[14] 郑凯, 夏勇, 温小英, 等.从伴生稀土磷矿中富集与提取稀土元素的研究进展[J].矿产保护与利用, 2017(5):93-98. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=1a5e31db-5fc2-46f2-80d9-ea618276f6c3
[15] Wang Y L, Liao W P, Li D Q. A solvent extraction process with mixture of CA12 and Cyanex272 for the preparation of high purity yttrium oxide from rare earth ores[J]. Separation and Purification Technology, 2011, 82:197-201.
[16] 卢勇.稀土资源提取分离技术研究进展[J].四川有色金属, 2019(3):3-6. http://d.old.wanfangdata.com.cn/Periodical/scysjs201903002
[17] 许晓芳, 谭全银, 刘丽丽, 等.稀土元素分离与提纯技术研究现状及展望[J].环境污染与防治, 2019, 41(7):844-851. http://d.old.wanfangdata.com.cn/Periodical/hjwryfz201907020
[18] 尹敬群.风化壳淋积型稀土矿浸出液中稀土离子的微生物吸附研究[D].南昌大学, 2012.
[19] 李永绣, 周新木, 刘艳珠, 等.离子吸附型稀土高效提取和分离技术进展[J].中国稀土学报, 2012, 30(3):257-264. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb201203001
[20] 黄小卫, 李建宁, 张永奇, 等.P204-P507在酸性硫酸盐溶液中对Nd3+和Sm3+的协同萃取[J].中国有色金属学报, 2008, 18(2):366-371. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb200802030
[21] 牟苗苗, 陈广, 罗兴.新型稀土萃取剂研究现状与进展[J].矿产保护与利用, 2015(4):73-78. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=77e982d2-aa2d-4a8a-bc12-15ff0bc7a339
[22] Wang L S, Yu Y, Huang X W, et al. Thermodynamics and kinetics of thorium extraction from sulfuric acid medium by HEH(EHP)[J]. Hydrometallurgy, 2014(150):167-172.
[23] 刘晶, 王运东.皂化P507-HCl-煤油体系萃取镨钕[J].化工学报, 2014, 65(1):264-270. http://d.old.wanfangdata.com.cn/Periodical/hgxb201401035
[24] Xiaowei Huang, Jinshi Dong, Liangshi Wang. Selective recovery of rare earth from ion-adsorption rare earth ores by stepwise extraction with HEH(EHP) and HDEHP[J]. Green Chemistry, 2017, 19(5):1345-1352.
[25] Ye Q, Li G H, Deng B N, et al. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204[J]. Separation and Purification Technology, 2019, 209:175-181.
[26] Sun P P, Kim D, Cho S. Separation of neodymium and dysprosium from nitrate solutions by solvent extraction with Cyanex272[J]. Minerals Engineering, 2018, 118:9-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=07ae6f420ab9f0f5e6b96ceb8c0671d6
[27] Wang J L, Liu X Y, Fu J S, et al. Novel extractant (2, 4-dimethylheptyl)(2, 4, 4'-trimethylpentyl)phosphinic acid (USTB-2) for rare earths extraction and separation from chloride media[J]. Separation and Purification Technology, 2019, 209:789-799.
[28] Mohammedi H, Miloudi H, Tayeb A, et al. Study on the extraction of lanthanides by a mesoporous MCM-41 silica impregnated with Cyanex 272[J]. Separation and Purification Technology, 2019, 209:359-367. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1b246bb4088bb3e3f2f7ee70f6fe054f
[29] 焦芸芬, 廖春发, 聂华平, 等.Cyanex272浸渍树脂吸萃重稀土机理[J].过程工程学报, 2009, 9(6):1099-1102. http://d.old.wanfangdata.com.cn/Periodical/hgyj200906011
[30] Jain A, Singh O V, Tandon S N. Extraction of lanthanoids and some associated elements by mono-(2-ethylhexyl)phosphoric acid and their separations[J]. Journal of Radio analytical and Nuclear Chemistry, 1991, 147(2):355-361.
[31] Shabani M B, Akagi T, Masuda A. Preconcentration of trace rare-earth elements in seawater by complexation with bis(2-ethylhexyl) hydrogen phosphate and 2-ethylhexyl dihydrogen phosphate adsorbed on a C18 cartridge and determination by inductively coupled plasma mass spectrometry[J]. Analytical Chemistry, 1992, 64(7):737-743.
[32] He Z Y, Zhang Z Y, Yu J X, et al. Column leaching process of rare earth and aluminum from weathered crust elution-deposited rare earth ore with ammonium salts[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(11):3024-3033. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgysjsxb-e201611027
[33] Chemical Book. Phosphoric Acid 2-Ethylhexyl Ester(2-Ethylhexyl Phosphate, Mono- and Di-Estermixture)[EB/OL].[2017-06-12]. https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1691494.htm.
[34] He Z Y, Zhang Z Y, Yu J X, et al. Behaviors of rare earth, aluminum and ammonium in leaching process of weathered crust elution-deposited rare earth ore[J]. Chinese Rare Earths, 2015, 36(6):18-24. http://d.old.wanfangdata.com.cn/Conference/9467202
[35] 何正艳, 张臻悦, 余军霞, 等.风化壳淋积型稀土矿浸取过程中稀土和铝及铵的行为研究[J].稀土, 2015(6):18-24. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xitu201506004
[36] 何正艳.风化壳淋积型稀土矿浸取机理及过程强化研究[D].中南大学, 2016.
[37] 石富.稀土冶金技术(第2版)[M].北京:冶金工业出版社, 2015:68-76.
[38] 池汝安, 王淀佐.稀土提取与选矿技术[M].北京:科学出版社, 1996.224.
[39] 潜美丽.铝对P507体系萃取稀土元素的影响[D].东北大学, 2010.
[40] 刘红, 张一敏, 刘涛, 等.皂化P204优化钒萃取工艺研究[J].有色金属(冶炼部分), 2016(5):17-21. http://d.old.wanfangdata.com.cn/Periodical/ysjs-yl201605005
[41] 史先菊.P204从高浓度含锌溶液中萃取锌的技术及机理研究[D].长沙: 中南大学, 2011.
[42] Yang X L, Qiu T S. Influence of aluminum ions distribution on the removal of aluminum from rare earth solutions using saponified naphthenic acid[J]. Separation and Purification Technology, 2017, 186(2):290-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5d4a665e3072cd48616da5a2aaf3268b
-



 下载:
下载: