Synthesis of Two Aromatic Xanthates and Their Flotation Properties on Chalcopyrite
-
摘要:
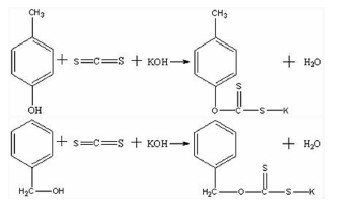
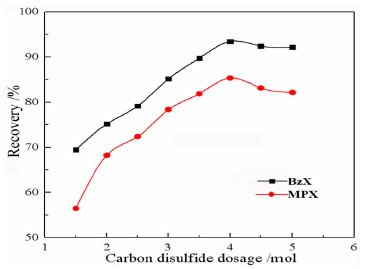
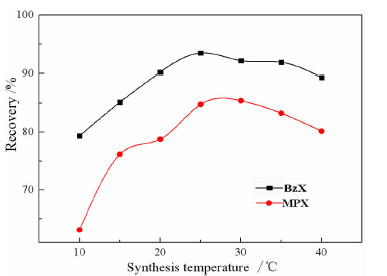
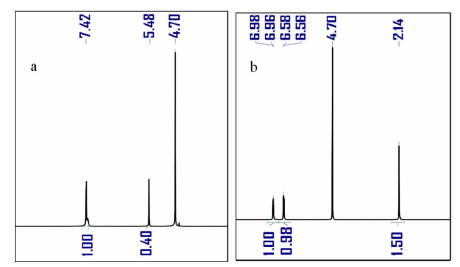
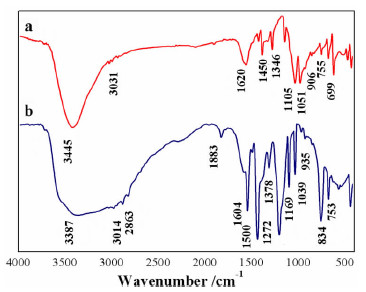
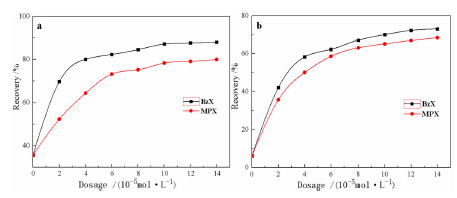
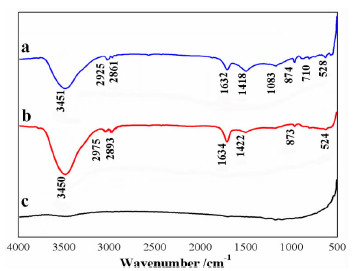
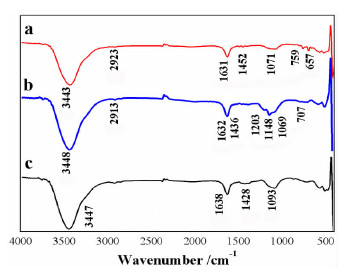
以苯甲醇、对甲基苯酚、二硫化碳和氢氧化钾为原料合成两种互为同分异构体的芳香基黄原酸盐——苯甲基黄原酸钾(BzX)和对甲基苯基黄原酸钾(MPX),并对其进行了结构表征。结果表明,二硫化碳与苯甲醇或对甲基苯酚优化的摩尔比为4 GA6FA 1,MPX优化的反应温度为30℃时,BzX优化的反应温度为25℃。采用单矿物浮选试验考查了两种药剂对黄铜矿的浮选性能,结果表明,BzX的浮选性能优于MPX。采用DFT计算和红外光谱分析考察了药剂在黄铜矿表面的吸附机理,证实了两种药剂在黄铜矿表面的吸附以化学吸附为主。
-
关键词:
- 芳香基黄原酸盐 /
- 同分异构体 /
- 浮选 /
- 黄铜矿 /
- [WTBZ]DFT计算
Abstract:Potassium benzyl xanthate (BzX) and potassium 4-methylphenyl xanthate(MPX), which are isomers of each other, were synthesized via the reaction of benzyl alcohol or p-methylphenol, carbon disulfide, and potassium hydroxide, and characterized by a variety of techniques. The results showed that the optimal molar ratio of carbon disulfide to benzyl alcohol or p-methylphenol was 4 GA6FA 1 and the optimal synthesis temperature for BzX was 30℃, and the optimal synthesis temperature for BzX was 25℃. The flotation performance and adsorption mechanism of BzX, MPX on the chalcopyrite were investigated. Flotation results and DFT theoretical calculations indicated that BzX exhibited better collecting performance compared with MPX. The adsorption mechanism of agents on the chalcopyrite surface was investigated by DFT calculation and infrared spectrum analysis. The results showed that the adsorption of the two collectors on the malachite surface was mainly chemical adsorption.
-
Key words:
- aromatic xanthate /
- isomers /
- flotation /
- chalcopyrite /
- DFT calculation
-

-
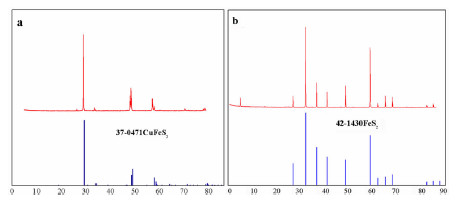
表 1 黄铜矿与黄铁矿中主要元素含量
Table 1. Element contents of chalcopyrite and pyrite
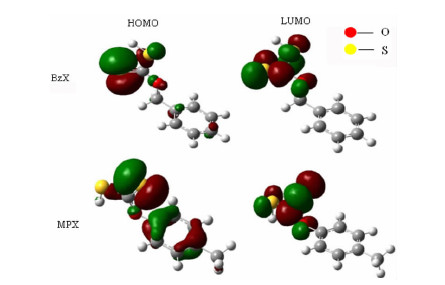
Elements Contents (%) Chalcopyrite Pyrite Cu 32.59 0.0917 Fe 27.84 48.70 S 29.23 47.27 Zn 0.103 0.122 Si 1.11 1.34 Al 0.213 0.235 表 2 在DFT/B3LYP6-311G(d)水平下捕收剂的前线轨道能量
Table 2. The frontier orbital eigenvalues of collectors at B3LYP/6-311+G(d) level
Reagent EHOMO ELUMO EHOMO—ELUMO BzX -0.24626 -0.06518 -0.18108 MPX -0.24895 -0.07044 -0.17851 -
[1] 朱一民,周菁.2017年浮选药剂的进展[J].矿产综合利用,2018(3):1-10. doi: 10.3969/j.issn.1000-6532.2018.03.001
[2] 梁爽,路亮,吴桂叶.硫化矿捕收剂的研究进展[J].中国矿业,2018,27(S2):156-158. http://d.old.wanfangdata.com.cn/Periodical/sxhg201210034
[3] Sha Liang, Xueyi Guo, Ningchuan Feng. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous[J]. Journal of Hazardous Materials, 2009, 170(1):425-429. doi: 10.1016/j.jhazmat.2009.04.078
[4] 罗忠岩.浮选药剂的国内外研究综述[J].当代化工研究,2018(11):54-55. doi: 10.3969/j.issn.1672-8114.2018.11.030
[5] Priyanka Dhar, Maria Thornhill, Hanumantha Rao Kota. Comparison of single and mixed reagent systems for flotation of copper sulphides from Nussir ore[J]. Minerals Engineering, 2019, 142:105930. doi: 10.1016/j.mineng.2019.105930
[6] 谢杰,胡春梅.国内外硫化铜镍矿选矿现状及未来发展方向[J].矿产保护与利用,2018(5):143-150. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2dc8f9c1-5ca0-4610-b11a-2e7a5dd10132
[7] Liuyin Xia, Brian Hart, Faïçal Larachi. Galvanic interaction of pyrite with Cu activated sphalerite and its effect on xanthate adsorption[J]. The Canadian Journal of Chemical Engineering, 2019, 97(10):2671-2677. doi: 10.1002/cjce.23562
[8] 王浩林,王礼平,王强强,等.硫化铜矿石选矿技术进展[J].金属矿山,2017(11):116-121. doi: 10.3969/j.issn.1001-1250.2017.11.024
[9] Shaojun Bai, Chunlong Li, Xianyu Fu. Promoting sulfidation of smithsonite by zinc sulfide species increase with addition of ammonium chloride and its effect on flotation performance[J]. Minerals Engineering, 2018, 125:190-199. doi: 10.1016/j.mineng.2018.03.040
[10] 韩巧凤,番寿龙,杨绪杰,等.芳香族黄原酸盐的合成及萃取性能研究[J].江苏化工,2002(3):33-34. http://d.old.wanfangdata.com.cn/Periodical/jshg200203008
[11] 李西山,朱一民.利用同分异构化学原理研究浮选药剂Y-89的同分异构体甲基异戊基黄药[J].湖南有色金属,2010(2):24-26. http://d.old.wanfangdata.com.cn/Periodical/hnysjs201002005
[12] P.K. Ackerman, G.H. Harris, R.R. Klimpel, et al. Evaluation of flotation collectors for copper sulfides and pyrite, III. Effect of xanthate chain length and branching[J]. International Journal of Mineral Processing, 1987, 21(1-2):141-156. doi: 10.1016/0301-7516(87)90011-1
[13] Mohamed El Khames Saad, Nejmeddine Rabaaoui, Elimame Elaloui, et al. Mineralization of p-methylphenol in aqueous medium by anodic oxidation with a boron-doped diamond electrode[J]. Separation and Purification Technology, 2016, 171:157-163. doi: 10.1016/j.seppur.2016.07.018
[14] 马鑫,王帅,钟宏.苄基三硫代碳酸钠的合成及其对黄铜矿的浮选性能[J].中国有色金属学报,2018,28(5):1067-1075. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb201805024
[15] Xin Ma, Shuai Wang, Hong Zhong. Effective production of sodium isobutyl xanthate using carbon disulfide as a solvent:Reaction kinetics, calorimetry and scale-up[J]. Journal of Cleaner Production, 2018, 200:444-453. doi: 10.1016/j.jclepro.2018.07.251
[16] 苏大伟,孙中溪,刘园园,等.不同链长黄原酸钾的制备和提纯[J].济南大学学报(自然科学版),2009,23(1):22-25. doi: 10.3969/j.issn.1671-3559.2009.01.006
[17] E.I. Finkelshtein, R.S. Shamsiev. Spectral and structural properties of carotenoids-DFT and thermochemical calculations[J]. Journal of Molecular Structure, 2019, 1197:583-593. doi: 10.1016/j.molstruc.2019.07.067
[18] 孙伟,杨帆,胡岳华,等.前线轨道在黄铜矿捕收剂开发中的应用[J].中国有色金属学报,2009,19(8):1524-1532. doi: 10.3321/j.issn:1004-0609.2009.08.027
[19] 王瑜,刘建,曾勇,等.量子化学计算在硫化铅锌矿浮选机理中的研究进展[J].矿产保护与利用,2018(3):37-42,48. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=a7e9103a-093c-4269-aada-a184a52627cf
[20] A. Sarvaramini, F. Larachi, B. Hart. Collector attachment to lead-activated sphalerite-experiments and DFT study on pH and solvent effects[J]. Applied Surface Science, 2016, 367:459-472. doi: 10.1016/j.apsusc.2016.01.213
[21] 曹飞,孙传尧,王化军,等.烃基结构对黄药捕收剂浮选性能的影响[J]. 北京科技大学学报,2014,36(12):1589-1594. http://d.old.wanfangdata.com.cn/Periodical/bjkjdxxb201412004
[22] 陈建华.浮选捕收剂的结构及其作用机理研究[J].矿产保护与利用,2017(4):98-106. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=62868a9f-46ba-4d99-b8dd-6006d13f0cf9
[23] E. Frimpong, A. Skelton, B. Honarparvar. DFT study of the interaction between DOTA chelator and competitive alkali metal ions[J]. Journal of Molecular Graphics and Modelling, 2017,76:70-76. doi: 10.1016/j.jmgm.2017.06.025
[24] Ilaria Ciofini, Claude A. Daul. DFT calculations of molecular magnetic properties of coordination compounds[J]. Coordination Chemistry Reviews, 2003, 238-239:187-209. doi: 10.1016/S0010-8545(02)00330-2
-



 下载:
下载: