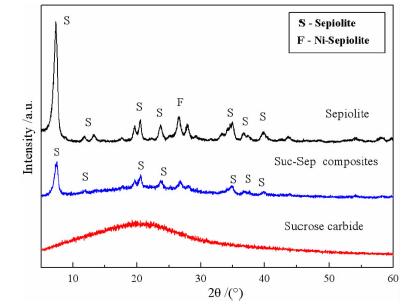
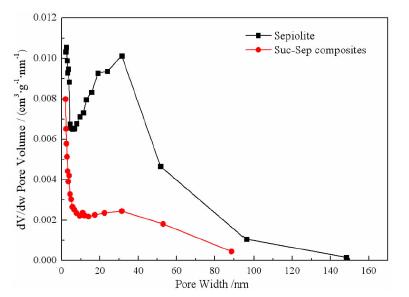
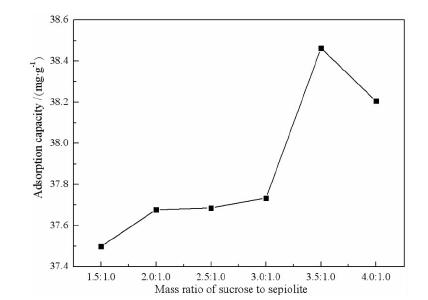
Preparation of Sucrose Carbide/Sepiolite Composite and the Optimization of Response Surface Methodology
-
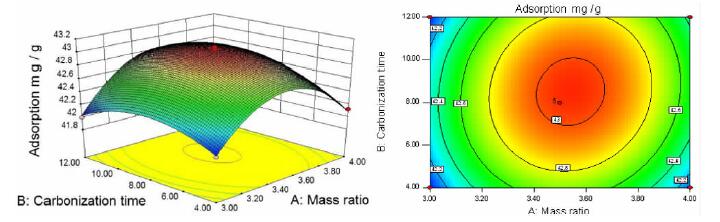
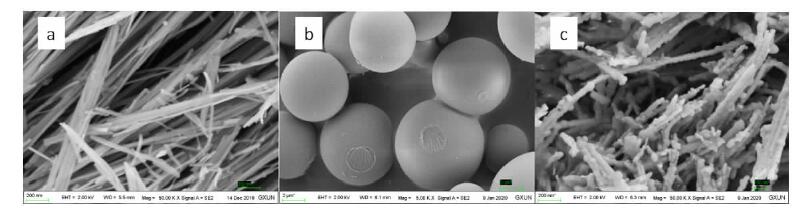
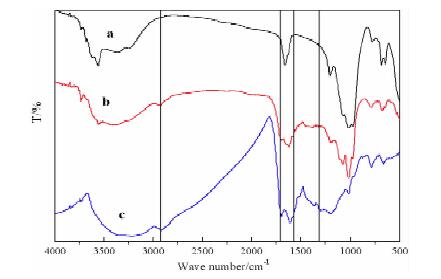
摘要: 以蔗糖和海泡石为实验原材料,利用水热碳化法制备出新型蔗糖碳化物/海泡石复合材料,冷冻真空干燥法干燥样品。利用XRD、IR、SEM、BET对样品进行表征。以亚甲基蓝作为吸附质,通过单因素和响应面法优化制备的工艺。综合实验结果表明,蔗糖碳化物能够成功的被负载到海泡石表面制备出蔗糖碳化物/海泡石复合材料,其制备最优工艺条件为:蔗糖与海泡石质量比为3.5:1.0,碳化时间为8 h,碳化温度为220℃,对亚甲基蓝的最优吸附量为42.983 mg/g;各因素对复合材料吸附亚甲基蓝性能的影响顺序为:蔗糖与海泡石质量比>碳化时间>碳化温度,并且发现复合材料对亚甲基蓝的吸附效果优于现有的文献报导值。Abstract: Using sucrose and sepiolite as experimental raw materials, a new sucrose carbide/sepiolite composite material was prepared by hydrothermal carbonization method, and the composite material was dried by freeze vacuum drying method. The composite samples were characterized by XRD, IR, SEM and BET, and methylene blue was selected as adsorbate to investigate its adsorption properties. The preparation technical conditions of the composite were optimized by the single factor experiment and the response surface methodology. The results showed that the optimum preparation conditions were as follows. Sucrose carbide could be successfully loaded on the surface of sepiolite to prepare sucrose carbide/sepiolite composite. The optimum adsorption capacity of methylene blue is 42.983 mg/g (composite) with the mass ratio of sucrose to sepiolite of 3.5:1.0, the carbonization time of 8 h, and the carbonization temperature of 220 ℃. The order of the influence of the factors on the adsorption properties of methylene blue was: mass ratio of sucrose to sepiolite > carbonization time > carbonization temperature. It could be found that the adsorption effect of composite materials on methylene blue was superior to the values reported in the existing literature.
-
Key words:
- sepiolite /
- sucrose /
- hydrothermal carbonization /
- composite
-

-
表 1 响应面试验因素水平设计表
Table 1. Design table of factors and levels of response surface experiments
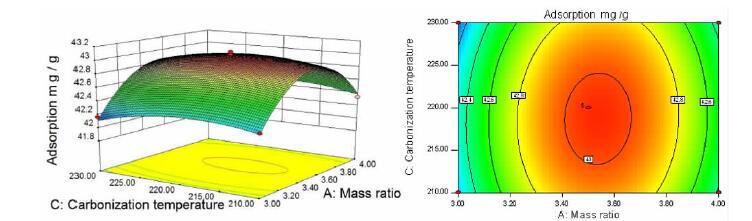
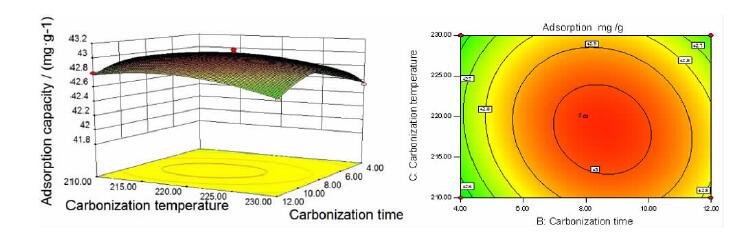
Factor Level -1 0 1 A 3.0:1.0 3.5:1.0 4.0:1.0 B/h 4 8 12 C/℃ 210 220 230 表 2 响应面试验设计及相对应响应值表
Table 2. Response surface experimental design table and corresponding response values
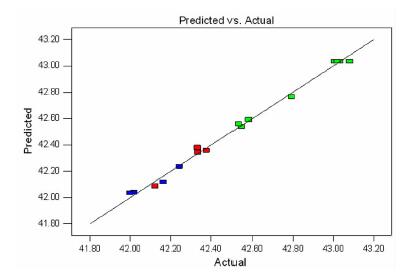
SN A/(g·g-1) B/h C/℃ Adsorption capacity/(mg·g-1) 1 0 0 0 43.081 7 2 0 -1 1 42.532 3 3 -1 -1 0 42.019 1 4 -1 0 -1 42.243 9 5 0 1 -1 42.793 2 6 1 -1 0 42.123 0 7 1 0 -1 42.333 0 8 1 0 1 42.250 3 9 0 -1 -1 42.547 2 10 0 1 1 42.581 1 11 -1 1 0 41.997 9 12 0 0 0 43.032 9 13 -1 0 1 42.163 3 14 0 0 0 43.005 3 15 0 0 0 43.020 1 16 1 1 0 42.165 4 17 0 0 0 43.035 0 表 3 响应面试验数据方差分析表
Table 3. Analysis of data variance of response surface experiments
Variance
sourceSum of
squaresdf Mean
squareF value P value Model 2.36 9 0.26 144.48 < 0.0001 significant A 0.069 1 0.069 38.14 0.0005 ** B 0.035 1 0.035 19.20 0.0032 ** C 0.012 1 0.012 6.42 0.0390 * AB 0.019 1 0.019 10.46 0.0144 * AC 0.00171 1 0.00171 0.94 0.3642 BC 0.009722 1 0.009722 5.35 0.0539 A2 1.65 1 1.65 906.01 < 0.0001 ** B2 0.33 1 0.33 182.42 < 0.0001 ** C2 0.084 1 0.084 46.13 0.0003 ** Residual 0.013 7 0.001816 Lack of Fit 0.009423 3 0.003141 3.82 0.1141 Not significant Pure Error 0.003289 4 0.0008224 Cor Total 2.37 16 注:表中*代表显著(P值<0.05);**代表极显著(P值<0.01)。 表 4 验证试验结果
Table 4. Results of verification experiments
SN Adsorption capacity/(mg·g-1) Average adsorption capacity/(mg·g-1) 1 42.897 2 43.069 42.983 3 42.982 -
[1] 鲁旖, 仇丹, 章凯丽.海泡石吸附剂的应用研究进展[J].宁波工程学院学报, 2016(1):17-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=668506081
[2] 李春生, 吴国霖, 徐传云.海泡石基催化材料的应用[J].中国非金属矿工业导刊, 2014(1):10-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgfjskgydk201401004
[3] 梁学峰.黏土矿物表面修饰及其吸附重金属离子的性能规律研究[D].天津市: 天津大学.2015.
[4] 王雪琴, 李珍, 杨友生, 等.海泡石的改性及应用研究现状[J].中国非金属矿工业导刊, 2003(3):11-14. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgfjskgydk200303003
[5] 李春生, 吴国霖, 徐传云.海泡石基催化材料的应用[J].中国非金属矿工业导刊, 2014(1):10-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgfjskgydk201401004
[6] 徐灵舒.凹凸棒粘土/碳复合材料的制备及其水污染处理应用初步研究[D].扬州市: 扬州大学.2013.
[7] 杨松, 黄辉, 龚青涛, 等.磁性/碳纳米复合材料的制备及其在污水处理方面的应用[J].化工技术与开发, 2018, 47(12):49-53. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxhg201812014
[8] 胡盛, 杜梦江, 周红艳, 等.凹凸棒石/C复合材料的制备及其吸附性能研究[J].非金属矿, 2018(4):99-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fjsk201804031
[9] Li M, Li W, Liu S, et al. Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method[J]. Journal of Materials Research, 2012, 27(8): 1117-1123. doi: 10.1557/jmr.2011.447
[10] 魏静, 褚云, 蒋国民, 等.水热碳化法制备碳微球[J].功能材料, 2014(S2):136-139. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gncl2014z2029
[11] Wang Y, Liu S, Hong X, et al. Microporous Carbon Materials Derived From Sucrose as Sulfur Host for Lithium Sulfur Batteries[J]. IOP Conference Series Materials Science and Engineering, 2018, 394(4):042031. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=IOP_9439836
[12] Bedin K C, André L. Cazetta, Souza I P A F, et al. Porosity enhancement of spherical activated carbon: Influence and optimization of hydrothermal synthesis conditions using response surface methodology[J]. Journal of Environmental Chemical Engineering, 2018, 6(1). http://www.sciencedirect.com/science/article/pii/S221334371730708X
[13] Yao C, Shin Y, Wang L Q, et al. Hydrothermal dehydration of aqueous fructose solutions in a closed system[J]. The Journal of Physical Chemistry C, 2007, 111(42): 15141-15145. doi: 10.1021/jp074188l
[14] Sevilla Solís, Marta, Fuertes Arias A B, et al. The production of carbon materials by hydrothermal carbonization of cellulose[J]. Carbon, 2009, 47(9): 2281-2289. doi: 10.1016/j.carbon.2009.04.026
[15] Cui X, Antonietti M, Yu S H, et al. Structural effects of iron oxide nanoparticles and iron ions on the hydrothermal carbonization of starch and rice carbohydrates[J]. Small, 2006, 2(6): 756-759. doi: 10.1002/smll.200600047
[16] Demir-Cakan R, Baccile N, Antonietti M, et al. Carboxylate-Rich carbonaceous materials via One-Step hydrothermal carbonization of glucose in the presence of acrylic acid[J]. Chemistry of Materials, 2009, 21(3): 484-490. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d6eca33f795a96820fc41db75cc6508e
[17] Qi Y, Zhang M, Qi L, et al. Mechanism for the formation and growth of carbonaceous spheres from sucrose by hydrothermal carbonization[J]. RSC Advances, 2016, 6: 102428. doi: 10.1039/C6RA21312J
[18] 李杨瑞.关于广西的甘蔗育种[J].广西糖业, 2019(3):3-9. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxzt201903001
[19] 李海云, 王永垒, 许涛, 等.水热碳化法合成蔗糖基碳材料的工艺研究[J].齐齐哈尔大学学报(自然科学版), 2018(5):47-53. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qqhrdxxb201805011
[20] 徐升, 方亮, 弓晓峰, 等.响应面分析法优化微波辅助硫酸亚铁改性海泡石制备工艺[J].功能材料, 2016(2):2235-2241. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gncl201602046
[21] 刘相廷, 李俊锋, 李培雅, 等.膨润土纳米片水凝胶的制备及其吸附性能研究[J].矿产保护与利用, 2019, 39(4):144-150, 158. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kcbhyly201904028
[22] Zhu P, Ren Z, Wang R, et al. Preparation and visible photocatalytic dye degradation of Mn-TiO2/sepiolite photocatalysts [J]. Frontiers of Materials Science, 2020, 14 (07): 33-42. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zggdxxxswx-clkx202001004
[23] Marrakchi F, Khanday W A, Asif M, et al. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16 [J]. International Journal of Biological Macromolecules, 2016: S0141813016307206. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ef7eec0dec12317e379c9034136c88f6
[24] 邢新艳, 陈得军, 赵东方, 等.C/海泡石复合吸附剂的水热法制备及其对水中亚甲基蓝的吸附研究[J].化工新型材料, 2013(2):115-117. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxxcl201302039
[25] Liang W, Meng-Lin C, Xing-Cun H E, et al. Adsorption of Methylene Blue onto Activated Sepiolite[J]. The Chinese Journal of Process Engineering, 2009, 9: 1095-1098. http://en.cnki.com.cn/article_en/cjfdtotal-hgyj200906013.htm
[26] Liu RR, Zhijiang J I, Wang J, et al. Mesocrystalline TiO2/sepiolite composites for the effective degradation of methyl orange and methylene blue[J]. Frontiers of Materials Science, 2018, 12(3):292-303. doi: 10.1007/s11706-018-0429-9
[27] Li J, Hong-Yan Z, Sheng X, et al. Facile preparation of sepiolite@LDH composites for the visible-light degradation of organic dyes[J]. Chinese Journal of Catalysis, 2018, 39(11):1832-1841. doi: 10.1016/S1872-2067(18)63120-1
-



 下载:
下载: