Study on Flotation of Copper Molybdenumt and Mechanism Under the Low Basicity Condition
-
摘要:
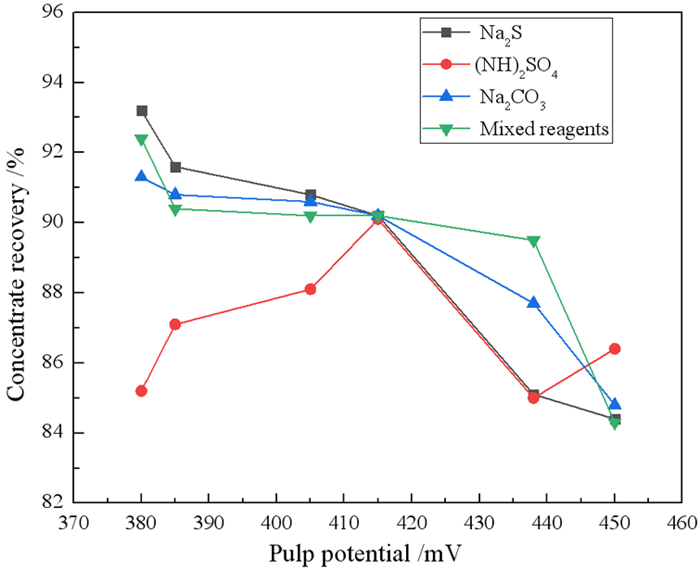
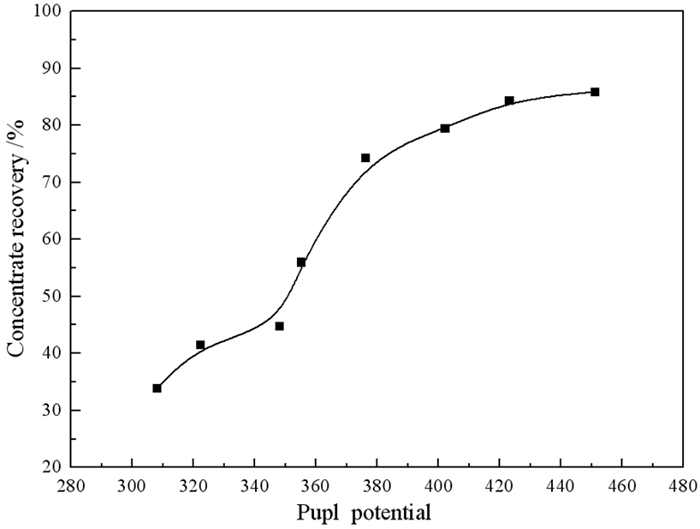
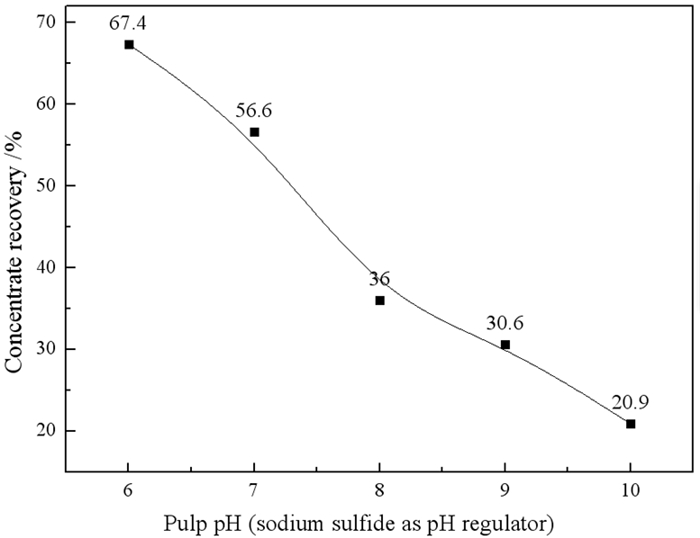
为探究矿浆电位对铜钼矿浮选的影响,采用黄铜矿、辉钼矿纯矿物作为样品,进行了矿浆pH、浮选药剂种类及用量对矿浆电位影响的研究。结果表明,黄铜矿上浮最佳矿浆电位为360 mV,pH为8。硫化钠、硫酸铵、碳酸钠三种调整剂按质量比1:1:1混合使用时,黄铜矿更易达到上浮电位区间。同时,当矿浆pH为9左右时,巯基乙酸能很好地抑制黄铜矿的上浮,并且对辉钼矿具有很好的选择性作用效果,有利于二者的分离。机理分析结果表明,在矿浆pH为8时,矿浆中生成大量的CuS是促进黄铜矿上浮的主要原因。
Abstract:In order to explore the influence of pulp potential on flotation of copper molybdenum ore, chalcopyrite and molybdenite were used as samples to study the effects of pulp pH, flotation reagent type and consumption on pulp potential. The results show that the optimum slurry potential is 360 mV and pH is 8. When sodium sulfide, ammonium sulfate and sodium carbonate are mixed in 1:1:1, chalcopyrite is easier to reach the floating potential range. At the same time, when the pulp pH is about 9, mercaptoacetic acid can well inhibit the flotation of chalcopyrite, and has a good selective effect on molybdenite, which is conducive to the separation of the two. In the mechanism analysis, it is pointed out that the main reason for the flotation of chalcopyrite is the formation of a large amount of CuS in the pulp at pH 8.
-
Key words:
- separation of copper molybdenum /
- kerosene /
- potential regulation
-

-
表 1 试验使用的主要药剂
Table 1. Main chemicals used in the test
Reagent name chemical formula purity Manufacturer ammonium sulphate (NH4)2SO4 Analytically pure 湖南汇虹试剂有限公司 sodium sulfide Na2S Analytically pure 上海统亚化工科技发展有限公司 terpineol oil - industrial products 株洲选矿药剂厂 sodium carbonate Na2CO3 Analytically pure 天津市大茂化学试剂厂 kerosene - industrial products - mercaptoacetic acid HSCH2COOH Chemical purity 天津市科密欧化学试剂开发中心 MIBC Analytically pure - 表 2 试验使用的主要设备
Table 2. Main equipment used in experiments
Equipment Model Manufactor Flotation Machine XFG hanging cell flotation machine 长春探矿机械厂 PH meter pHSJ-4A 上海雷磁仪器厂 Calomel electrode Type 232 上海精密科学仪器有限公司 Platinum electrode Type 213 上海精密科学仪器有限公司 表 3 硫化钠在水中的反应式及平衡电位方程式
Table 3. reaction formula and equilibrium potential equation of sodium sulfide in water
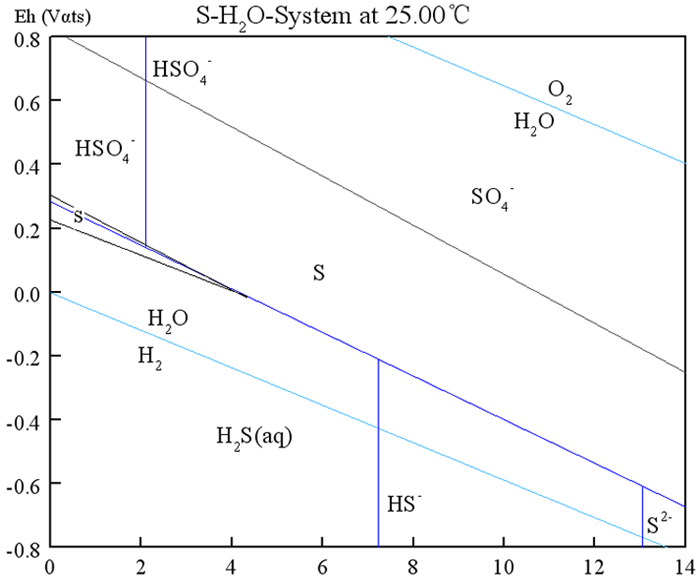
Serial number Chemical reaction formula Equilibrium equation 1 H2S↔HS-+H+ pH=7.02 2 HS-↔H++S2- pH=13.9 3 SO42-+H+↔HSO4- pH=1.9 4 S2-+4H2O=SO42-+8H4+8e Eh=0.157-0.059pH 5 H2S+4H2O=SO42-+10H++8e Eh=0.303-0.074pH 6 HS-+4H2O=SO42-+10H++8e Eh=0.252-0.066pH 7 H2S+4H2O=HSO4-+9H++8e Eh=0.289-0.066pH 8 H2S=S0+2H++2e Eh=0.230-0.059pH 9 HS-=S0+H++2e Eh=0.0232-0.0295pH 10 S2-=S0+2e Eh=-0.3876-0.059pH 11 S+4H2O=HSO4-+7H++6e Eh=0.309-0.069pH 12 S+4H2O=SO42-+8H++6e Eh=0.328-0.0787pH 表 4 黄铜矿-硫化钠-水体系下Eh-pH关系式
Table 4. Eh pH relationship in chalcopyrite-sodium sulfide-water system
Chemical reaction formula Equilibrium equation 1 CuS=Cu2++S+2e Eh=0.594+0.0295lg[Cu2+] 2 Cus+2H2O=Cu(OH)2+S+2H++2e Eh=0.853-0.059pH 3 CuFeS2+4H2O=CuS+Fe2++SO42-+8H++8e Eh=0.256-0.059pH 4 CuS+4H2O=Cu2++SO42-+8H++8e Eh=0.340-0.059pH 5 CuFeS2+7H2O=CuS+Fe(OH)3+SO42-+11H++9e Eh=0.373-0.072pH 6 CuS+6H2O=Cu(OH)2+SO42-+10H++8e Eh=0.449-0.0738pH -
[1] 张丽荣, 印万忠, 丁亚卓, 等.辉钼矿电位调控浮选试验研究[J].矿冶, 2008, 17(4):15-18. doi: 10.3969/j.issn.1005-7854.2008.04.004
[2] ZHIXIANG C, GUOHUA G, SHUANGKE L, et al. The Effect of Seaweed Glue in the Separation of Copper-Molybdenum Sulphide Ore by Flotation[J]. Minerals, 2018, 8(2):41-45.
[3] 赵敏捷.硫化铜矿电化学调控浮选应用与研究进展[J].矿冶, 2016, 25(5):15-18. doi: 10.3969/j.issn.1005-7854.2016.05.004
[4] 耿连胜.控制矿浆电位提高铜浮选回收率的研究[J].矿业快报, 2001(9):13-15.
[5] 史玲.无捕收剂电化学浮选技术研究[J].中国钼业, 2004, 28(6):19-22. doi: 10.3969/j.issn.1006-2602.2004.06.005
[6] PLACKOWSKI, C., W.J. BRUCKARD, A.V. Nguyen. Surface characterisation, collector adsorption and flotation response of enargite in a redox potential controlled environment[J]. Minerals Engineering, 2014, 65: 61-73.
[7] 孙传尧, 王福良, 师建忠, 等.蒙古额尔登特铜矿的电化学控制浮选研究与实践[J].矿冶, 2001, 10(1):20-26.
[8] V·帕那亚托夫, 魏明安, 肖力子, 等.铜锌浮选的电化学处理技术[J].国外金属矿选矿, 2000(11):39-40.
[9] 赵美法, 巯基乙酸的生产及应用[J].中国氯碱, 2004(6):15-16. doi: 10.3969/j.issn.1009-1785.2004.06.007
[10] 秦伟程, 巯基乙酸技术进展与发展趋势[J].广西化工, 2002(1):30-32.
[11] 胡岳华, 王淀佐, 浮选溶液化学[M].1988, 长沙: 湖南科学技术出版社.132-324.
-




 下载:
下载: