Study on Preparation and Photocatalytic Degradation Kinetics of Black TiO2/Kaolinite Composite
-
摘要:
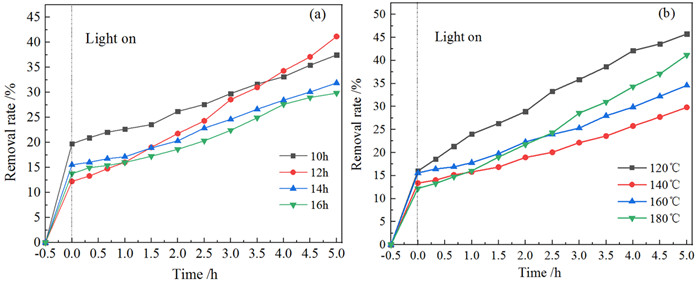
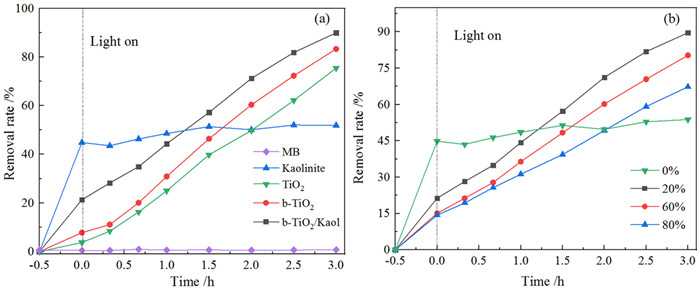
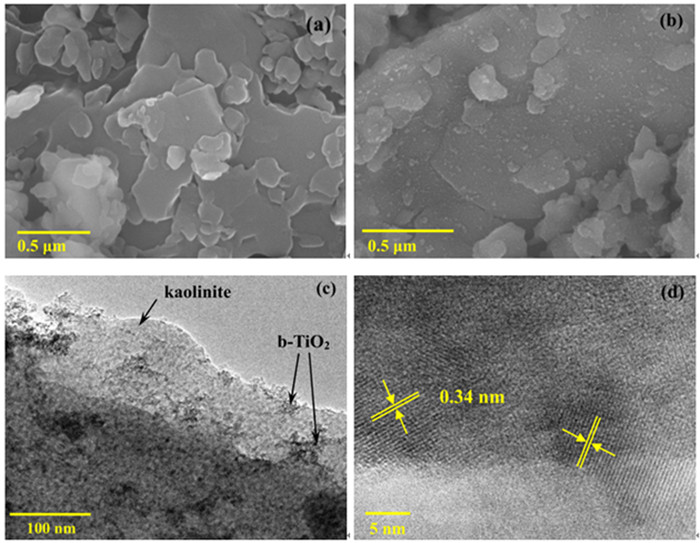
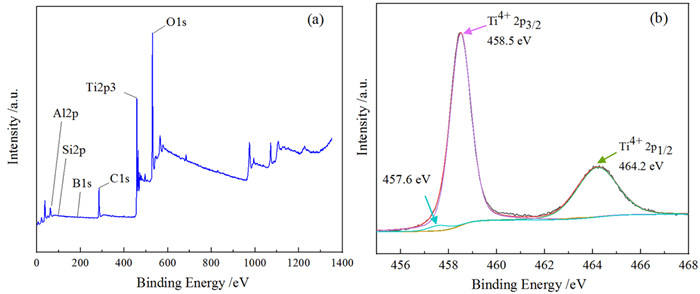
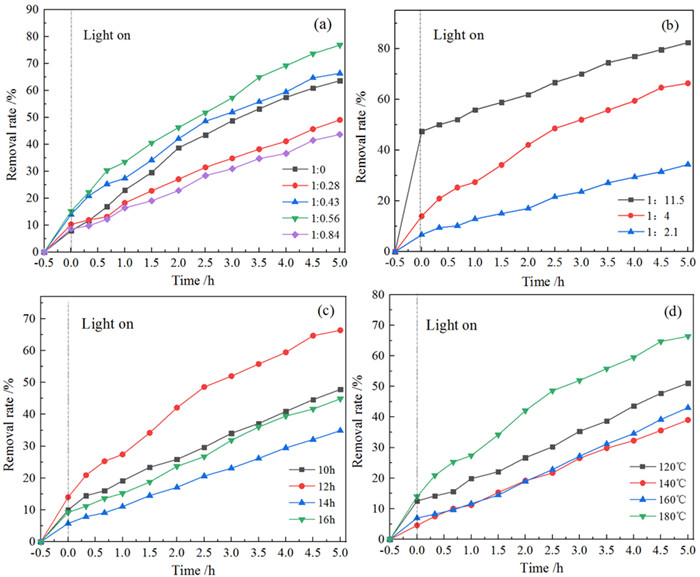
黑色TiO2作为一种新型光催化材料被广泛关注,但利用黏土改性黑色TiO2来提升其应用性能却鲜有报道。以高岭石为原料,钛酸四丁酯为前驱体,通过溶剂热法合成黑色TiO2/高岭石(b-TiO2/Kaol)光催化剂。采用扫描电镜(SEM)、透射电镜(TEM)和X-射线光电子能谱(XPS)技术对材料微观形貌及表面结构进行表征。研究了制备条件、负载量对材料可见光催化性能的影响,并结合动力学模型考察了亚甲基蓝(MB)初始pH值对去除效果的影响。结果表明,当摩尔比n(Ti4+):n(BH4-)为1:0.56、n(HNO3):n(H2O)为1:11.5、合成时间为12 h、水热合成温度为180℃时,制备黑色TiO2(b-TiO2)光催化活性最强;b-TiO2负载量为20%制得的b-TiO2/Kaol具有最佳的光催化性能,对MB的去除率达89.7%,光催化降解过程符合一级动力学模型。MB溶液初始pH为5~7更有利于MB的去除。Ti3+的掺杂使得b-TiO2光响应范围扩大,高岭石改性b-TiO2增强了b-TiO2/Kaol的吸附能力,两者协同促进了光催化性能的提高。
Abstract:As a new photocatalytic material, black TiO2 (b-TiO2) has been widely concerned. However, there are few reports on the investigation of the b-TiO2 modified by clay to improve its application performance. Black TiO2/kaolinite(b-TiO2/Kaol) photocatalyst was synthesized by solvothermal method using kaolinite as raw material and tetrabutyltitanate as precursor. The microstructure and surface structure of the materials were characterized by SEM, TEM and XPS. The effects of preparation conditions and loading rate on the photocatalytic performance of the materials were studied, and the effect of initial pH value of MB on the removal efficiency was investigated by combining with the kinetic model. The results showed that the best photocatalytic activity of b-TiO2 could be obtained with the n(Ti4+): n(BH4-) of 1:0.56, n(HNO3): n(H2O) of 1:11.5, synthesis time of 12 h and temperature of 180 ℃. And the photocatalytic activity of b-TiO2/Kaol was the best when the loading rate of b-TiO2 was 20%, and the removal rate of MB could reach 89.7%. The photodegradation process of MB by b-TiO2/Kaol accorded with the first-order kinetic model. It was more conducive to the removal of MB with the initial pH of MB from 5 to 7. The doping of Ti3+ broadened the photoresponse range of b-TiO2, and the b-TiO2 modified by the kaolinite enhanced the adsorption capacity of b-TiO2/Kaol, which both promoted the improvement of photocatalytic performance.
-
Key words:
- black TiO2 /
- kaolinite /
- solvothermal method /
- photocatalytic activity /
- kinetics
-

-
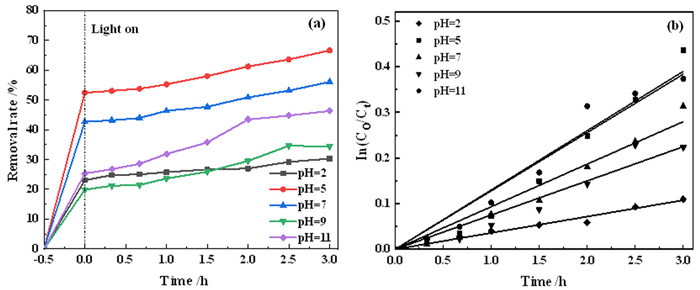
表 1 不同MB初始pH对应的降解方程及参数
Table 1. Degradation equation and parameters corresponding to different initial pH of MB
MB初始pH 光催化降解方程 k/min-1 t1/2/h R2 2 lnCt=2.0926-0.0358t 0.0358 19.36 0.9866 5 lnCt=1.5699-0.1279t 0.1279 5.42 0.9714 7 lnCt=1.7738-0.0932t 0.0932 7.44 0.9792 9 lnCt=2.1361-0.0749t 0.0749 9.25 0.9726 11 lnCt=2.0926-0.1299t 0.1299 5.34 0.9830 -
[1] CHEN X, LIU L, YU P Y, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331(6018): 746-750. doi: 10.1126/science.1200448
[2] GE J J, DU G H, KALAM A, et al. Oxygen vacancy-rich black TiO2 nanoparticles as a highly efficient catalyst for Li-O2 batteries[J]. Ceramics International, 2021, 47: 6965-6971. doi: 10.1016/j.ceramint.2020.11.045
[3] HAN L J, SU B T, LIU G, et al. Synthesis of oxygen vacancy-rich black TiO2 nanoparticles and the visible light photocatalytic performance[J]. Molecular catalysis, 2018, 456: 96-101. doi: 10.1016/j.mcat.2018.07.006
[4] NALDONI A, ALLIETA M, SANTANGELO S, et al. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles[J]. Journal of the American Chemical Society, 2012, 134(18): 7600-7603. doi: 10.1021/ja3012676
[5] SUN B J, ZHOU W, LI H Z, et al. Magnetic Fe2O3/mesoporous black TiO2 hollow sphere heterojunctions with wide-spectrum response and magnetic separation[J]. Applied Catalysis B: Environmental, 2018, 221: 235-242. doi: 10.1016/j.apcatb.2017.09.023
[6] JO WK, YOO HJ. Combination of ultrasound-treated 2D g-C3N4 with Ag/black TiO2 nanostructure for improved photocatalysis[J]. Ultrasonics Sonochemistry, 2018, 42: 517-525. doi: 10.1016/j.ultsonch.2017.12.019
[7] CAO Y, XING Z P, HU M Q, et al. Mesoporous black N-TiO2-x hollow spheres as efficient visible-light-driven photocatalysts[J]. Journal of Catalysis, 2017, 356: 246-254. doi: 10.1016/j.jcat.2017.10.023
[8] DUAN Y Y, ZHANG M, WANG L, et al. Plasmonic Ag-TiO2-x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies[J]. Applied Catalysis B: Environmental, 2017, 204: 67-77. doi: 10.1016/j.apcatb.2016.11.023
[9] ZHOU G, SHEN L, XING Z, et al. Ti3+ self-doped mesoporous black TiO2/graphene assemblies for unpredicted-high solar-driven photocatalytic hydrogen evolution[J]. Journal of colloid and interface science, 2017, 505: 1031-1038. doi: 10.1016/j.jcis.2017.06.097
[10] SW A, JC A, JM A, et al. Defective black Ti3+ self-doped TiO2 and reduced graphene oxide composite nanoparticles for boosting visible-light driven photocatalytic and photoelectrochemical activity[J]. Applied Surface Science, 2019, 467/468: 45-55. doi: 10.1016/j.apsusc.2018.10.138
[11] HU P W, YANG H M. Insight into the physicochemical aspects of kaolins with different morphologies[J]. AppliedClay Science, 2013, 74: 58-65. http://www.sciencedirect.com/science/article/pii/S016913171200261X
[12] LI X Y, PENG K, CHEN H X, et al. TiO2 nanoparticles assembled on kaolinites with different morphologies forefficient photocatalytic performance[J]. Scientific Reports, 2018, 8: 11663-11. doi: 10.1038/s41598-018-29563-8
[13] NIU J N, WU A C, WANG D X, et al. Coloading of TiO2 and C3N4 on kaolinite nanotubes for obviously improved photocatalytic performance in degradation of methylene blue dye[J]. Materials Letters, 2018, 230: 32-35. doi: 10.1016/j.matlet.2018.07.073
[14] HOANG S, GUO S, HAHN N T, et al. Visible light driven photoelectrochemical water oxidation on nitrogen-modified TiO2 nanowires[J]. Nano Letters, 2012, 12(1): 26-32. doi: 10.1021/nl2028188
[15] 因博, 王际童, 徐伟, 等. 中孔炭负载二氧化钛光催化剂的制备及降解甲基橙[J]. 新型炭材料, 2013, 28(1): 47-54. https://www.cnki.com.cn/Article/CJFDTOTAL-XTCL201301012.htm
[16] HOFFMANN M R, MARTIN S T, CHOI W, et al. Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95(1): 69-96. doi: 10.1021/cr00033a004
-



 下载:
下载: