Experimental Study on Comprehensive Recovery of Molybdenum, Sulfur and Copper from Alkaline Leaching Molybdenum Sludge
-
摘要:
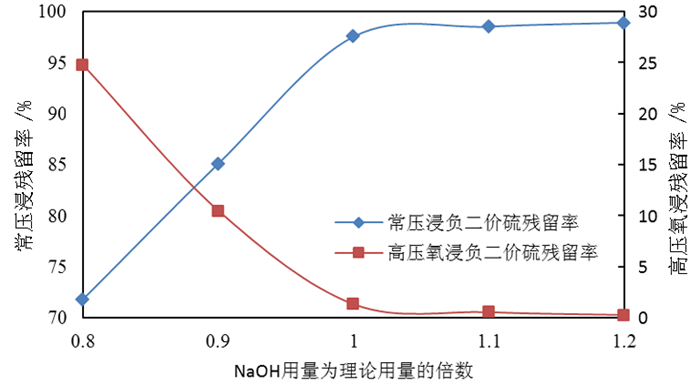
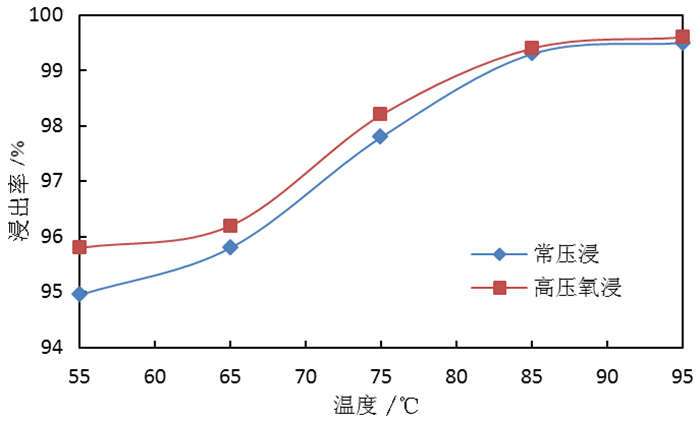
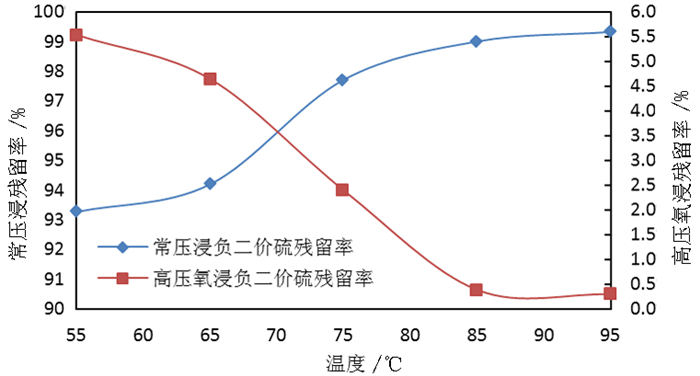
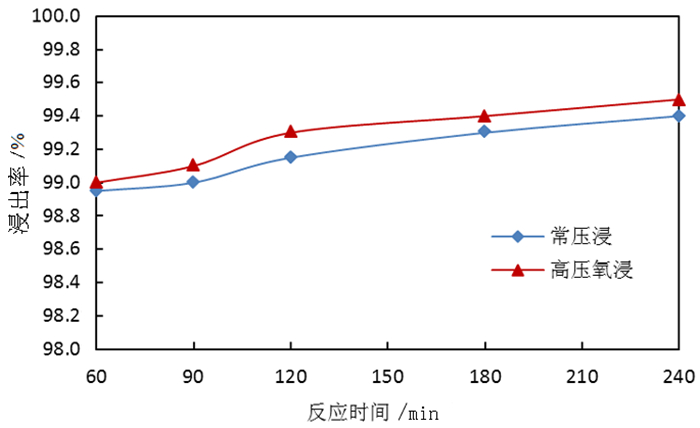
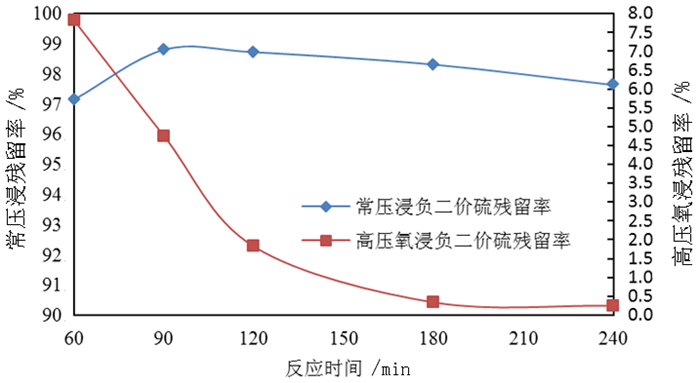
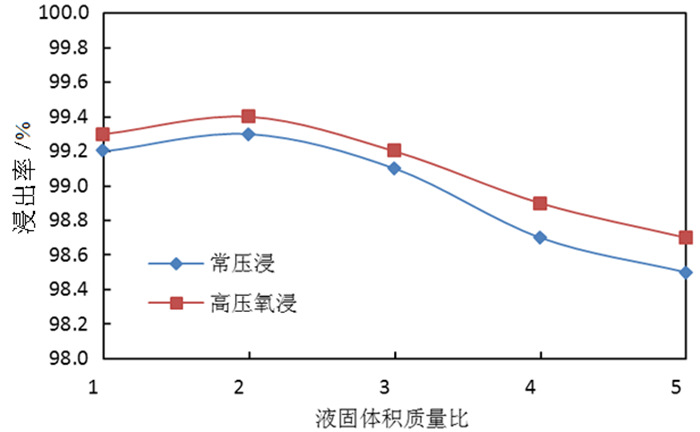
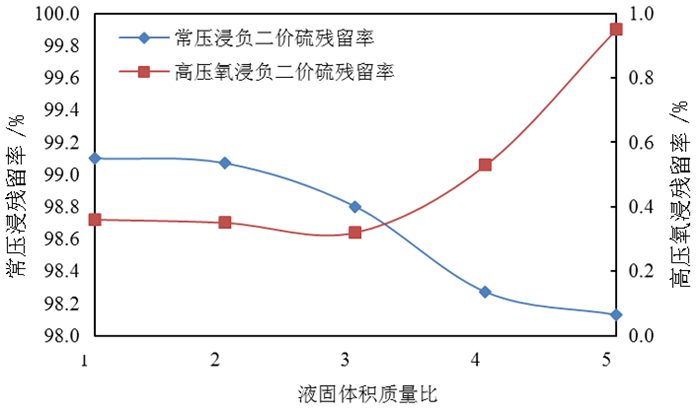
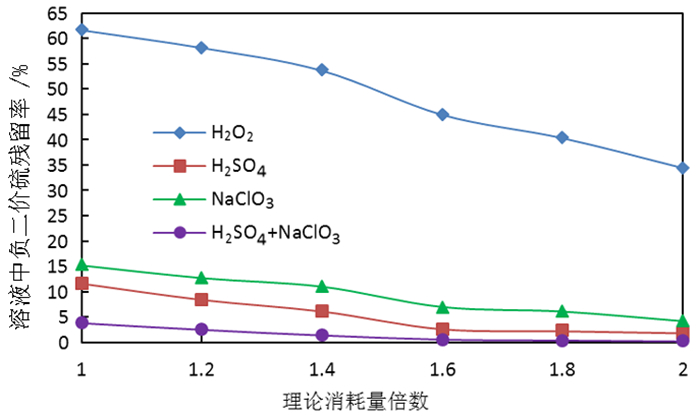
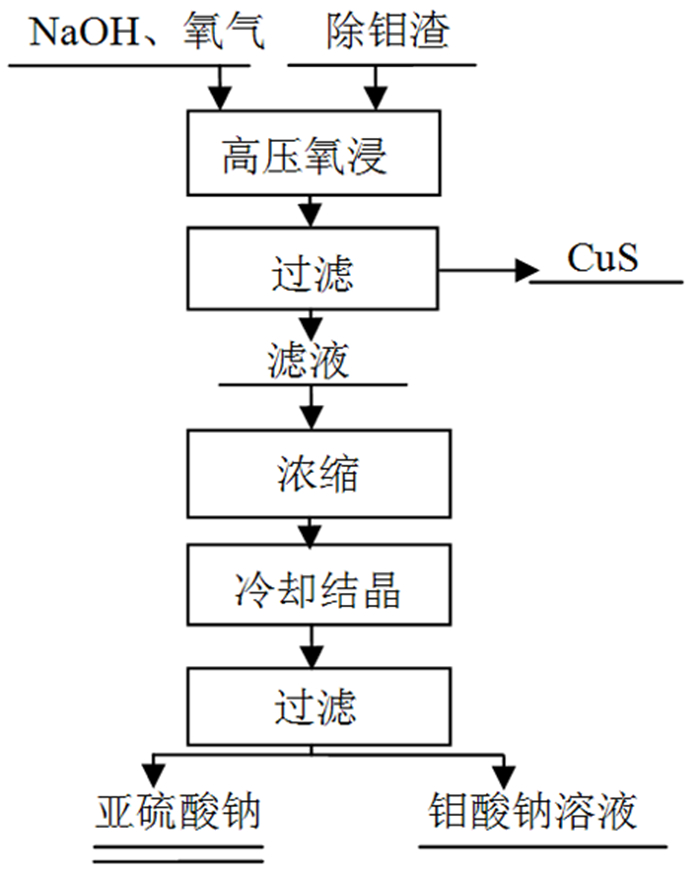
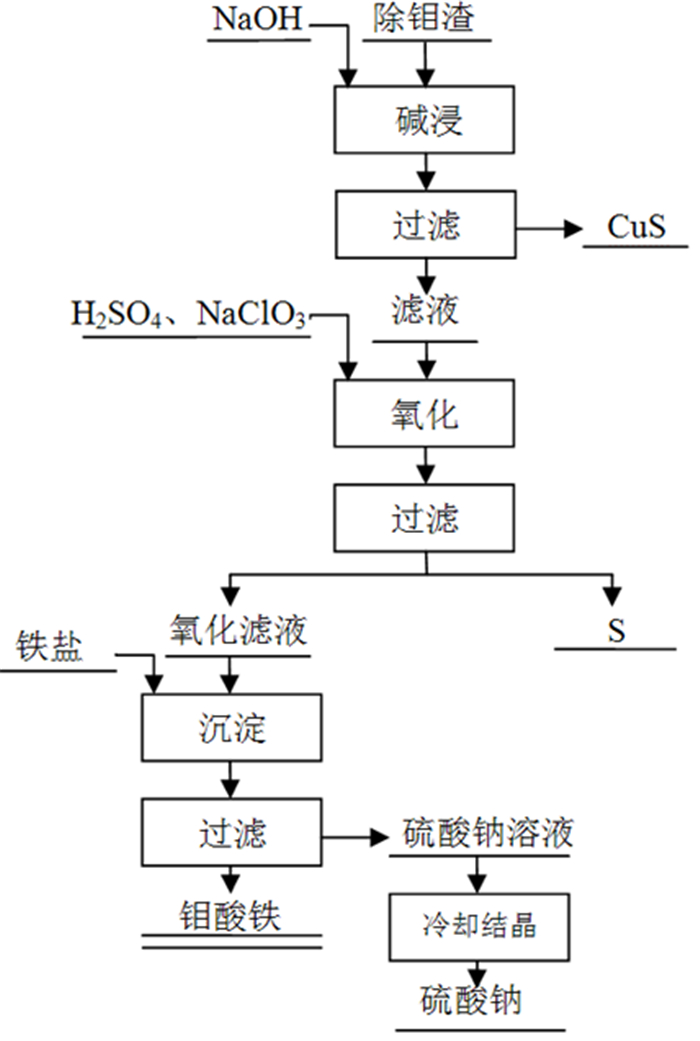
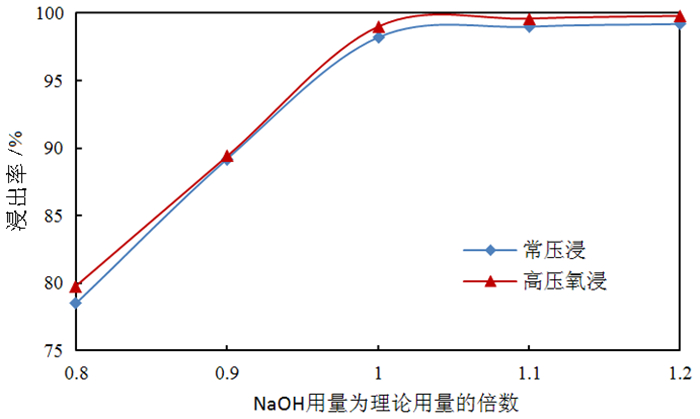
为了综合回收钨冶炼除钼渣中的钼、硫、铜,提出碱浸除钼渣分离铜和钼,氧化浸出液中S2-以分离硫和钼的思路,并对比了常压碱浸和高压氧碱浸两种工艺,详细考察碱浸过程氢氧化钠用量、温度、反应时间,液固比等工艺条件对钼浸出率、S2-残留率的影响规律。试验结果表明,常压碱浸在温度85℃、氢氧化钠用量为理论量1.1倍、反应180 min、液固体积质量比3 GA6FA 1时,钼浸出率为99.48%,铜浸出率低于0.1%,S2-残留率高于98%,选用硫酸与氯酸钠氧化碱浸滤液可实现S2-残留率低于0.2%。高压氧碱浸在温度85℃、氢氧化钠用量为理论量1.1倍、反应180 min、液固体积质量比3 GA6FA 1时,钼浸出率99.82%,铜浸出率低于0.5%,S2-残留率低至0.35%;两种工艺均可实现钼与铜、硫的深度分离,为除钼渣的综合利用提供切实可行的方案。
Abstract:In order to comprehensively recover molybdenum, sulfur, and copper from molybdenum removal residues from tungsten smelting, this paper proposes the idea of alkaline leaching molybdenum removal residues to separate copper and molybdenum, and oxidation of S2- in the leaching solution to separate sulfur and molybdenum. This article investigates in detail the influence of sodium hydroxide dosage, temperature, reaction time, liquid-solid ratio and other process conditions on the molybdenum leaching rate and S2-residual rate in the alkaline leaching process. The test results show that when the normal pressure alkaline leaching temperature is 85 ℃, the sodium hydroxide dosage is 1.1 times the theoretical amount, the reaction is 180 minutes, and the liquid-solid mass ratio is 3 GA6FA 1, the molybdenum leaching rate is 99.48%, and the copper leaching rate is less than 0.1% The S2-residual rate is higher than 98%. When sulfuric acid and sodium chlorate are used as oxidants to oxidize the alkali leaching filtrate, the S2-residual rate is lower than 0.2%. When high-pressure oxygen alkaline leaching is at a temperature of 85 ℃, the amount of sodium hydroxide is 1.1 times the theoretical amount, the reaction is 180 minutes, and the mass ratio of liquid to solid is 3 GA6FA 1, the molybdenum leaching rate is 99.82%, the copper leaching rate is less than 0.5%, and the S2-residual rate As low as 0.35%; both processes can achieve deep separation of molybdenum from copper and sulfur, providing a practical solution for the comprehensive utilization of molybdenum removal sludge.
-

-
表 1 除钼渣的主要化学成分
Table 1. The main chemical composition of molybdenum removal sludge
/% 元素 Mo Cu S W 除钼渣 12.46 21.71 32.34 1.44 -
[1] 霍广生, 赵中伟, 李洪桂, 等. 不同金属硫化物从钨酸盐溶液中除钼的效果[J]. 中国有色金属学报, 2004, 14(2): 302-305. doi: 10.3321/j.issn:1004-0609.2004.02.027
[2] 霍广生. 钨冶炼过程中钨钼分离新工艺及其理论研究[D]. 长沙: 中南大学, 001.76.
http://cdmd.cnki.com.cn/article/cdmd-10533-2002060683.htm [3] 肖超, 吴海国. 陈化除钼渣处理工艺试验研究[J]. 中国钼业, 2012, 36(6): 25-28. doi: 10.3969/j.issn.1006-2602.2012.06.006
[4] 黄美萍, 朱同浩. 从硫化除钼沉淀渣中回收钨的试验[J]. 中国钨业, 1992(7): 18-19. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU199207003.htm
[5] 付维琴, 杨大锦. 从除钼渣中浸出钨钼试验研究[J]. 湿法冶金, 2015, 34(3): 193-195. https://www.cnki.com.cn/Article/CJFDTOTAL-SFYJ201503007.htm
[6] 龚丹丹, 黄泽辉, 赵立夫, 等. 钼渣处理工艺试验研究[J]. 中国钨业, 2015(3): 38-42. doi: 10.3969/j.issn.1009-0622.2015.03.009
[7] 孙铭, 王嘉, 江英英, 等. APT铜钼渣回收工艺过程及环境影响分析[J]. 广东化工, 2012, 39(7): 145-147. doi: 10.3969/j.issn.1007-1865.2012.07.078
[8] 贾利攀, 刘旭恒等. 除钼渣浸出行为的热力学分析[J]. 稀有金属, 2019, 43(5): 526-531. https://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS201905011.htm
[9] 唐忠阳. 从除钼渣中回收铜、钼新工艺的研究[D]. 长沙: 中南大学, 2002.
http://cdmd.cnki.com.cn/article/cdmd-10533-2006036810.htm [10] 李洪桂, 羊建高, 李昆. 钨冶金学[M]. 长沙: 中南大学出版社, 2010.217.
[11] 张义忠. 钨钼分离理论及新工艺研究[D]. 昆明: 昆明理工大学, 2002.
https://d.wanfangdata.com.cn/thesis/Y482305 [12] 朱传贵, 等. 钼渣中有价金属的回收. 中国钼业, 2000, 24(2): 40-41 doi: 10.3969/j.issn.1006-2602.2000.02.015
[13] 向铁根, 杨伯华. 钼冶金[M]. 长沙: 中南大学出版社, 2009.
[14] 汪洋. 从硫废料中回收相的生产实践. 中国钼业, 1999, 23(2): 30-31.
[15] 李洪桂. 稀有金属冶金学. 北京: 冶金工业出版社. 1989.
[16] 黄万抚. 从铜精矿中回收铂的试验研究. 中国钨业. 15(2): 21-23
http://www.cnki.com.cn/Article/CJFDTotal-ZGWU200002007.htm [17] 张邦胜, 蒋开喜, 等. 酸性加压氧化分解辉钼精矿的试验研究[J]. 稀有金属, 2007, 31(3): 384-392. doi: 10.3969/j.issn.0258-7076.2007.03.021
[18] 杨立斌, 杜娟, 沙作良, 等. 十水硫酸钠冷却结晶动力学的研究[J]. 无机盐工业. 2009.41(4): 18-20. doi: 10.3969/j.issn.1006-4990.2009.04.006
[19] 任倩, 刘锦锐, 杨伟. 一种从除钼渣中提取钼制钼酸钠的方法: CN108893611B[P]. 2020-01-21.
[20] 刘锦锐, 任倩, 杨伟. 一种以除钼渣为原料制取钼酸铁的方法: CN 108977672 B[P]. 2020-09-25.
-



 下载:
下载: