Study on Selective Leaching of Thallium Bearing Zinc Ash
-
摘要:
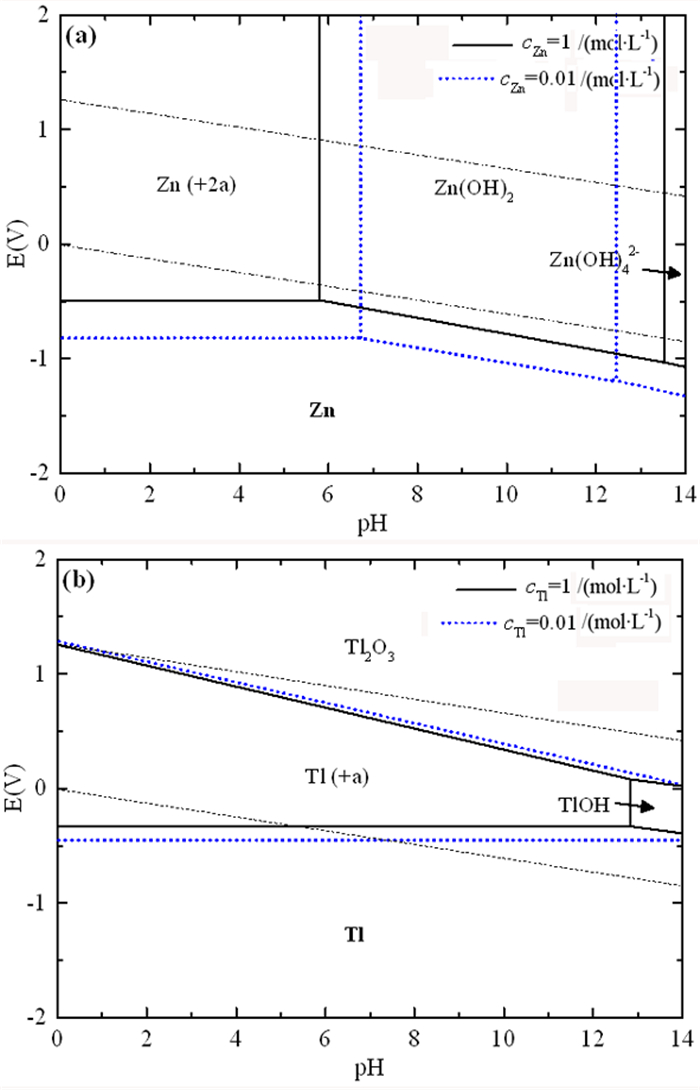
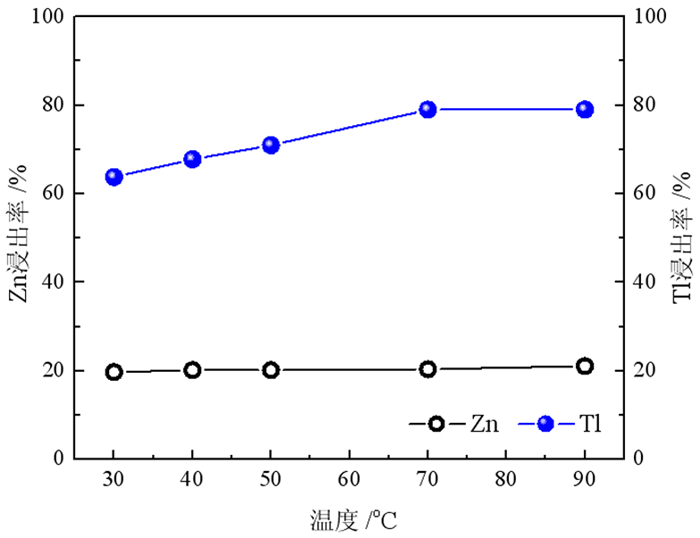
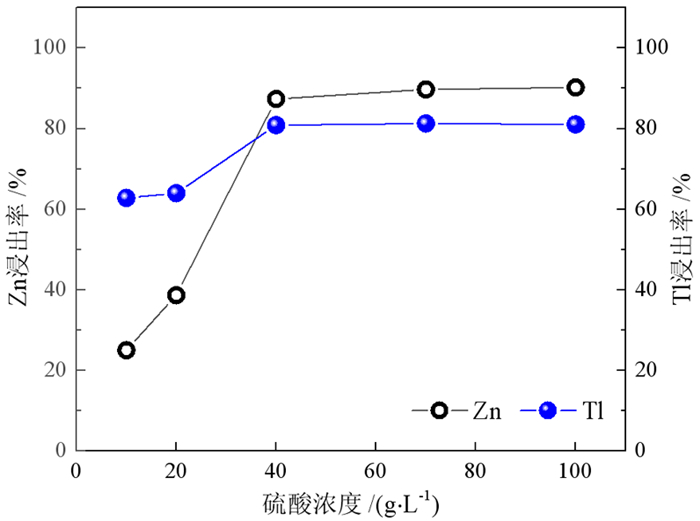
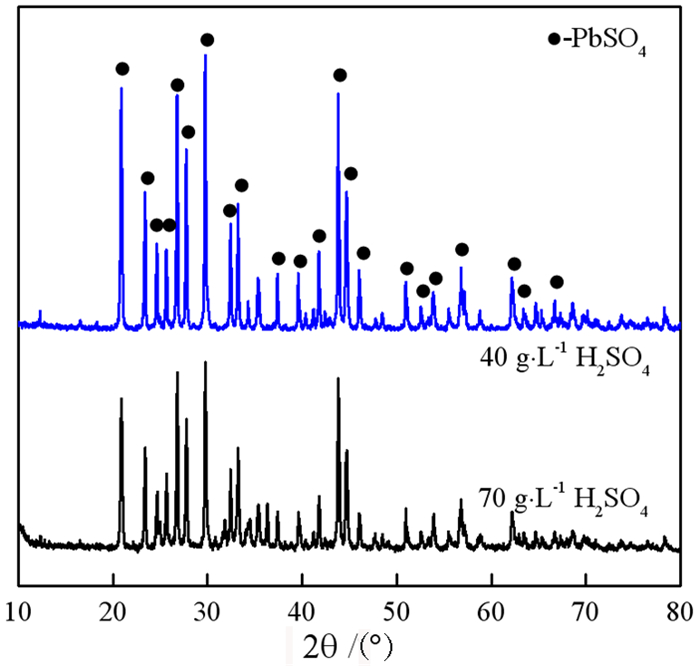
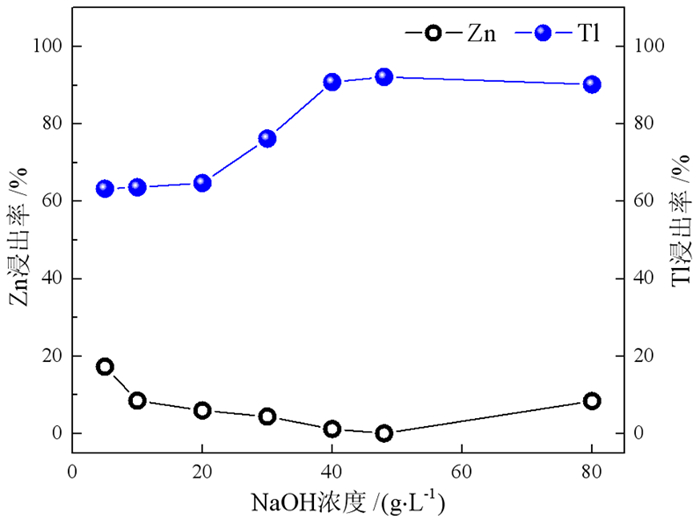
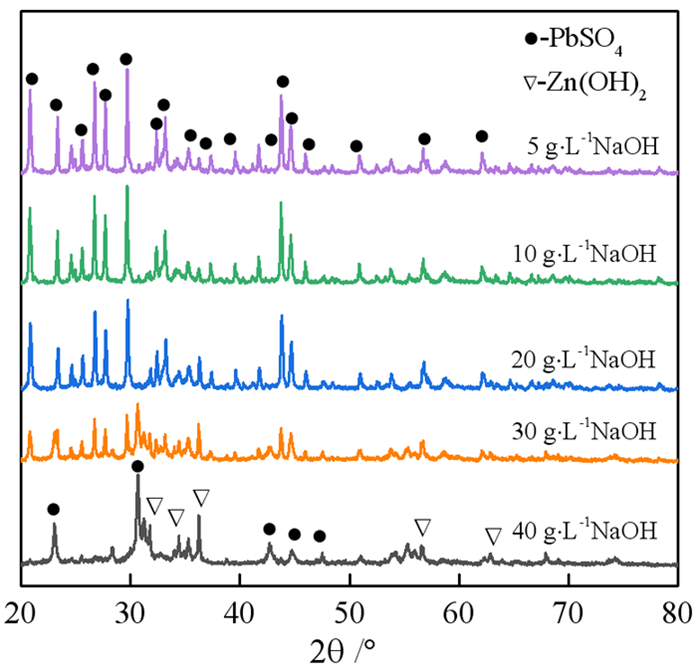
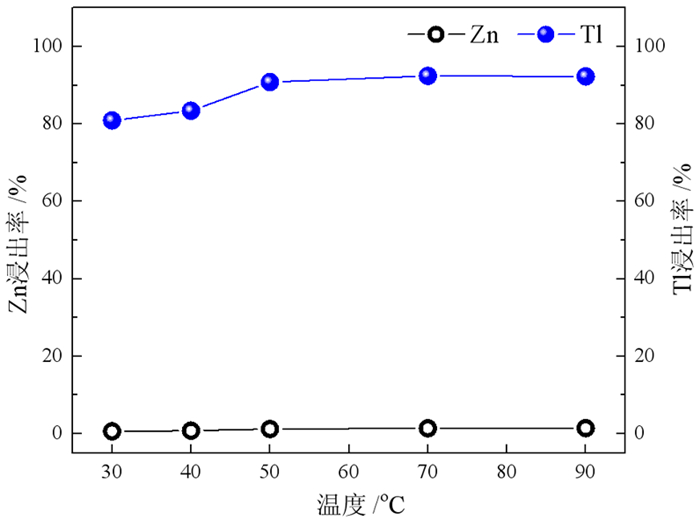
在铅锌冶炼渣挥发处理过程中产生含铊锌烟灰,是铊在冶炼中的主要富集物。采用水浸、H2SO4浸出和NaOH浸出分别处理锌烟灰,研究了酸碱浓度和浸出温度对锌和铊浸出率的影响。结果表明,水浸时铊浸出率随着温度升高而缓慢提高,在70℃时可达78%左右;酸浸选择性不好,酸浸时酸度和温度提高均会增加锌和铊的浸出率,在硫酸浓度40 g·L-1、温度70℃的条件下铊和锌浸出率分别达79%和85%以上;碱浸铊具有良好的选择性,铊的浸出率随碱浓度增加而提高,在NaOH 40 g·L-1、温度70℃的优化条件下,铊和锌的浸出率分别为91%和1%左右。最终选定碱浸工艺处理含铊烟灰,通过对碱性浸出液的硫化沉淀、硫酸浸出和锌板置换得到纯度为92.84%海绵铊。新工艺实现了对超低铊含量烟灰的资源化利用和开路除铊,具有工艺简捷、选择性好的优点。
Abstract:In the smelter of lead and zinc, the thallium bearing zinc ash was yielded during the volatilization of lead and zinc slag, and it is the main enrichment of thallium. In this paper, water, H2SO4 and NaOH solution were used to leach the ash, and the effects of concentration and temperature on the leaching of zinc and thallium were investigated. The results show that the leaching rate of thallium increases slowly with the increasing of temperature, and reached 78% above 70 ℃. The selectivity of acidity leaching for zinc and thallium was poor. The leaching rate of thallium and zinc reached 79% and 85% under the conditions of H2SO4 concentration of 40 g·L-1 and temperature of 70 ℃. While the alkalinity leaching has an excellent selectivity for thallium and zinc, the leaching rate of thallium increases with the increasing of alkali concentration. Under the optimum conditions of NaOH 40 g·L-1 and temperature 70 ℃, the leaching rates of thallium and zinc are about 91% and 1%, respectively. Therefore, alkaline leaching was used for leaching of Tl bearing ash. The product thallium, with a purity of 92.84%, was obtained by sulfide precipitation, H2SO4 leaching and Zn plate replacement of lixivium. The new process achieves the resource utilization and open-circuit of thallium from ultra-low thallium ash, and has the advantages of simple process and good selectivity.
-
Key words:
- zinc hydrometallurgy /
- zinc ash /
- thallium /
- alkaline leaching /
- acidic leaching
-

-
表 1 锌烟灰中元素含量
Table 1. Chemical composition of zinc ash
/% 元素 Zn O Pb S Na 含量 31.86 25.8 18.47 8.58 3.57 元素 Cl Fe Cd F Tl 含量 3.54 3.52 2.2 0.98 0.43 表 2 锌烟灰中锌的物相组成形式
Table 2. Chemical phase composition of Zn in Zn -bearing ash
/% 锌物相 硫酸锌 氧化锌 硫化锌 硅酸锌 铁酸锌 占比 18.42 49.00 17.70 7.63 7.25 表 3 选择性碱浸出后液成分
Table 3. Solution composition of the lixivium after selective alkaline leaching
/(g·L-1) 成分 Zn Cd Tl pH 浓度 0.0005 0.0305 0.7224 8.5 表 4 金属铊制备过程条件及结果
Table 4. Process conditions and results of the metallic Tl preparation
硫化沉淀 溶液初始pH 温度/℃ 时间/h Na2S理论倍数 沉铊率/% 8.5 30 0.5 4 99.92 8.5 40 0.5 4 99.91 8.5 70 0.5 4 99.87 硫酸浸出 液固比 温度/℃ 时间/h 硫酸浓度
/(g·L-1)浸出率/% 5:1 80 5 60 84.61 5:1 80 5 100 95.36 锌板置换 初始Tl浓度
/(g·L-1)温度/℃ 时间/h 硫酸浓度
/(g·L-1)置换率/% 85 80 8 5 99.82 表 5 海绵铊元素含量分析
Table 5. Chemical composition of the spongy Tl
/% 元素 Tl Cd O Zn S 含量 92.84 4.56 1.27 0.445 0.243 元素 Si Cl Cu Fe Al 含量 0.04 0.032 0.0255 0.025 0.014 -
[1] GALVAN-ARZATE S, SANTAMARIA A. Thallium toxicity [J]. Toxicology Letters, 1998, 99(1): 1-13. doi: 10.1016/S0378-4274(98)00126-X
[2] BELZILE N, CHEN Y W. Thallium in the environment: acritical review focused on natural waters, soils, sedi-ments and airborne particles [J]. Applied Geochemis-try, 2017, 84: 218-243. doi: 10.1016/j.apgeochem.2017.06.013
[3] 谭欣, 王中明, 肖巧斌, 等. 银铅无碱混选工艺分选含银低品位铅锌硫化矿石[J]. 矿产保护与利用, 2021(2): 89-98. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2fa74b8d-b058-405d-9540-10540266260e
[4] 敖顺福. 典型铅锌选矿厂废水零排放工艺对比分析[J]. 矿产保护与利用, 2021(1): 38-44. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=260f7f81-fd8f-4e18-8b3b-f12425818d7b
[5] 李凯旋, 冷成彪, 任志, 等. 铅锌矿伴生的稀散元素研究进展[J]. 矿物学报, 2021, 41(3): 225-233. https://www.cnki.com.cn/Article/CJFDTOTAL-KWXB202103001.htm
[6] GENCHI GIUSEPPE, CAROCCI ALESSIA, LAURIA GRAZIANTONIO, et al. Thallium use, toxicity, and detoxification therapy: an overview[J]. Applied Sciences, 2021, 11(18): 1-15.
[7] 陈玲玲, 韩俊伟, 覃文庆, 等. 铅锌冶炼渣综合利用研究进展[J]. 矿产保护与利用, 2021(3): 49-54. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=37a4e951-b923-422d-9dfd-b3084b776bb7
[8] 陈永亨, 谢文彪, 吴颖娟, 等. 中国含铊资源开发与铊环境污染[J]. 深圳大学学报, 2001(1): 57-63. doi: 10.3969/j.issn.1000-2618.2001.01.013
[9] 林文军, 刘一宁, 赵为上, 等. 氧化锌烟灰的综合回收方法: CN101818254A[P]. 2010-09-01.
[10] 王雷. 含锌烟灰回收锌的工艺研究[J]. 烧结球团, 2020, 45(3): 67-71. https://www.cnki.com.cn/Article/CJFDTOTAL-SJQT202003014.htm
[11] 徐素鹏, 李晓乐, 汤长青, 等. 用铅锌烟灰和氯碱厂副产盐酸制备纳米氧化锌的清洁工艺[J]. 无机盐工业, 2017, 49(11): 50-53. https://www.cnki.com.cn/Article/CJFDTOTAL-WJYG201711014.htm
[12] 邵传兵, 鲁兴武, 马爱军, 等. 萃取法从含铊烟尘中综合回收稀散金属的研究[J]. 有色金属(冶炼部分), 2014, 4(10): 57-58+63. doi: 10.3969/j.issn.1007-7545.2014.10.014
[13] 林文军, 王文军, 刘一宁, 等. 综合回收含镉铟高氯氧化锌的试验研究[J]. 湖南有色金属, 2016, 32(5): 24-26+33. doi: 10.3969/j.issn.1003-5540.2016.05.007
[14] 成林, 田学达, 张小云, 等. 浮选方铅矿精矿中铊的脱除工艺[J]. 中国有色金属学报, 2018, 28(2): 425-433. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201802025.htm
[15] HAIYIN XU, YUANLING LUO, PING WANG, et al. Removal of thallium in water/wastewater: A review[J]. Water Research, 2019, 165: 114981. doi: 10.1016/j.watres.2019.114981
[16] YING HUANG, DIYUN CHEN, LINGJUN KONG, et al. Aqueous two-phase systems (polyethylene glycol + ammonia sulfate) for thallium extraction: Optimization of extraction efficiency, structural characterization, and mechanism exploration[J]. Separation and Purification Technology, 2020, 235: 115740. doi: 10.1016/j.seppur.2019.115740
[17] 周令治, 陈少纯. 稀散金属提取冶金[M]. 北京: 冶金工程出版社, 2008: 109-110.
-




 下载:
下载: