Research Progress on Desilication Technology and Flotation Reagent Regime of Iron Ore
-
摘要:
硅是铁矿石中一种典型的有害杂质,降低铁精矿中硅含量一直是铁矿石分选的重要课题。对铁矿石脱硅工艺与浮选药剂制度的研究进展做了系统的综述,重点介绍了浮选脱硅的研究现状和发展趋势。文中指出含硅铁矿石钙离子活化—阴离子反浮选工艺具有广阔的应用前景,进一步提高浮选药剂的选择性和适应性具有重要的现实意义。
Abstract:Silicon is a typical harmful impurity in iron ore., So reducing the content of silicon in iron concentrate is always an important topic in iron ore separation.This paper systematically summarizes the research progress of iron ore desilication technology and flotation reagent regime.The research status and development trend of flotation desilication are introduced emphatically.It points out that calcium ion activation-anion reverse flotation of iron ore containing silicon has broad application prospects and how to further improve the selectivity and adaptability of reagents is of great practical significance to the process.
-
Key words:
- iron ore /
- desilication process /
- anion reverse flotation
-

-
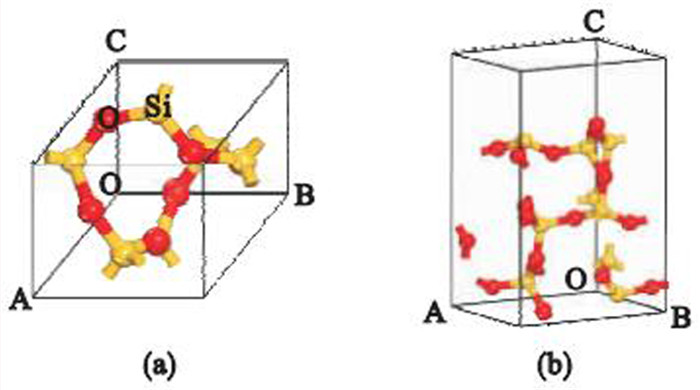
图 1 石英晶体结构[77]
Figure 1.
表 1 国外常用胺类捕收剂[54]
Table 1. Common amine collectors abroad
商品名称 化学名称 饱和度 Flotigam SA-B 十八酰胺醋酸盐 C1215%C1620%C1865% Flotgiam T2A-B 牛脂丙烯胺 C125%C1630%C1865% Collector 075/94 脂肪丙烯二胺 — HOE F2835-B 醚二胺醋酸盐 C12C13 Flotigam EDA-B 醚胺醋酸盐 C10 Flotigam EDA-3B 醚胺醋酸盐 C10 MG-70-A5 醚胺醋酸盐 C18~10烃氧基 MG-83-A 醚二胺醋酸盐 — MG-98-A3 醚胺醋酸盐 — ECNA 04D 醚胺 C12C13 Nb 104 缩合胺 C18 Nb 112 缩合胺 C8~10胺、C18缩合胺 Colmin C12 醚胺醋酸盐 — Poliad A-3 醚胺醋酸盐 — -
[1] FILIPPOV L O, FILIPPOVA I V, SEVEROV V V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates[J]. Minerals Engineering, 2010, 23(2): 91-98. doi: 10.1016/j.mineng.2009.10.007
[2] 邹忠平, 项钟庸, 王刚. 《高炉炼铁工程设计规范》[C]. 中国钢铁年会暨宝钢学术年会. 2015.
[3] YUAN S, ZHOU W, HAN Y, et al. Individual enrichment of manganese and iron from complex refractory ferromanganese ore by suspension magnetization roasting and magnetic separation[J]. Powder Technology, 2020, 373: 689-701. doi: 10.1016/j.powtec.2020.07.005
[4] 李博琦, 谢贤, 纪翠翠, 等. 鞍山地区贫磁铁矿选矿工艺试验[J]. 矿产综合利用, 2020(4): 93-99. doi: 10.3969/j.issn.1000-6532.2020.04.015
[5] 李亮, 李晓波, 徐宝金. 某低品位难选磁铁矿石选矿试验[J]. 现代矿业, 2020, 36(9): 142-144. doi: 10.3969/j.issn.1674-6082.2020.09.036
[6] 宛彦鑫, 马越, 胡海宽, 等. 新疆某贫铁矿石磁选试验研究[J]. 现代矿业, 2020, 36(10): 89-92. doi: 10.3969/j.issn.1674-6082.2020.10.027
[7] 张毅, 余莹, 张五志, 等. 鞍钢某铁尾矿磁化焙烧—磁选试验研究[J]. 金属矿山, 2021(7): 142-145. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202107021.htm
[8] 张小龙, 韩跃新, 李艳军, 等. 大西沟菱铁矿石磁化焙烧—弱磁选试验研究[J]. 金属矿山, 2016(12): 22-26. doi: 10.3969/j.issn.1001-1250.2016.12.006
[9] 袁帅, 韩跃新, 李艳军, 等. 国外某赤铁矿石悬浮磁化焙烧—磁选试验[J]. 金属矿山, 2018(8): 70-72. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201808014.htm
[10] 柯佳焱, 石云良, 肖金雄, 等. 俄罗斯某铁矿选矿工艺研究[J]. 矿冶工程, 2019, 39(6): 50-53. doi: 10.3969/j.issn.0253-6099.2019.06.012
[11] SKR A, DN B, SSRA B. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation[J]. Powder Technology, 2020, 367: 796-808. doi: 10.1016/j.powtec.2020.04.047
[12] RAYAPUDI V. Optimization of microwave carbothermal reduction for processing of banded hematite jasper ore[J]. Minerals Engineering, 2019, 138: 204-214. doi: 10.1016/j.mineng.2019.05.004
[13] RATH S S, SAHOO H, DHAWAN N, et al. Optimal Recovery of Iron Values from a Low Grade Iron Ore using Reduction Roasting and Magnetic Separation[J]. Separation Science & Technology, 2014, 49(12): 1 927-1 936.
[14] MA M. Froth Flotation of Iron Ores[J]. International Journal of Mining Engineering & Mineral Processing, 2012, 1(2): 56-61.
[15] HOUOT R. Beneficiation of phosphatic ores through flotation: Review of industrial applications and potential developments[J]. International Journal of Mineral Processing, 1982, 9(4): 353-384. doi: 10.1016/0301-7516(82)90041-2
[16] 陈雯, 余永富. 铁矿石选矿近十年来技术进步[C]//中国冶金矿山企业协会(Metallurgical Mines'Association of China). 2013中国冶金矿山科技大会会刊. 北京: 中国冶金矿山企业协会, 2013: 15.
[17] 马鸣泽. 磁铁矿对微细粒级赤铁矿浮选的影响及其机理研究[D]. 昆明: 昆明理工大学, 2019.
[18] 宋国君. 大红山赤褐铁矿型次级精矿浮选提质试验研究[D]. 昆明: 昆明理工大学, 2018.
[19] 刘文宝. 羟丙基胺类捕收剂的合成及在铁矿石反浮选中的应用研究[D]. 沈阳: 东北大学, 2015.
[20] MA X, MARQUES M, GONTIJO C. Comparative studies of reverse cationic/anionic flotation of Vale iron ore[J]. International Journal of Mineral Processing, 2011, 100(3/4): 179-183.
[21] FILIPPOV L O, SEVEROV V V, FILIPPOVA I V. An overview of the beneficiation of iron ores via reverse cationic flotation[J]. International Journal of Mineral Processing, 2014, 127: 62-69. doi: 10.1016/j.minpro.2014.01.002
[22] HOUOT R. Beneficiation of iron ore by flotation — Review of industrial and potential applications[J]. International Journal of Mineral Processing, 1983, 10(3): 183-204. doi: 10.1016/0301-7516(83)90010-8
[23] FATMA H, ELRAHIEM A. Recent Trends in Flotation of Fine Particles[J]. Journal of Mining World Express, 2014, 3: 63-69. doi: 10.14355/mwe.2014.03.009
[24] 吴红, 齐美超, 李保健, 等. 张庄铁矿石提铁降硅选矿试验及超纯铁精矿探索试验[J]. 现代矿业, 2019, 35(6): 144-148. doi: 10.3969/j.issn.1674-6082.2019.06.042
[25] 廖祥, 刘艳杰, 许蕊, 等. 福建某超贫磁铁矿弱磁精反浮选提铁降硅试验[J]. 金属矿山, 2013(5): 75-77. doi: 10.3969/j.issn.1001-1250.2013.05.020
[26] TOHRY A, DEHGHANI A. Effect of sodium silicate on the reverse anionic flotation of a siliceous-phosphorus iron ore[J]. Separation & Purification Technology, 2016, 164: 28-33.
[27] 勾金玲, 徐望华. 梅山铁矿铁精矿降硅选矿工艺试验[J]. 现代矿业, 2013, 29(7): 26-28+42. doi: 10.3969/j.issn.1674-6082.2013.07.007
[28] 宋乃斌. 齐大山铁矿石选矿技术研究综合评述[J]. 金属矿山, 2007(3): 1-5. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS200703000.htm
[29] 刘动. 反浮选应用于铁精矿提铁降硅的现状及展望[J]. 金属矿山, 2003(2): 38-42. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS200302012.htm
[30] HAN K N, HEALY T W, FUERSTENAU D W. Mechanism of Adsorption of Fatty Acids and Other Surfactants at the Oxide-Water Interface[J]. Journal of Colloid and Interface Science, 1973, 44(3): 407-414. doi: 10.1016/0021-9797(73)90316-0
[31] HUKKI R T, VARTIANEN O. An investigation of collecting effects of fatty acids in tall oil on oxide minerals particularly Ilemenite. AIChE Symposium Series, 1953, 71: 124-133.
[32] QUAST, KEITH. Literature review on the use of natural products in the flotation of iron oxide ores[J]. Minerals Engineering, 2017, 108: 12-24. doi: 10.1016/j.mineng.2017.01.008
[33] QUAST K B. A review of hematite flotation using 12-carbon chain collectors[J]. Minerals Engineering, 2000, 13(13): 1 361-1 376. doi: 10.1016/S0892-6875(00)00119-9
[34] ABHYARTHANA P, VENUGOPAL R. Investigation of adsorption mechanism of reagents (surfactants) system and its applicability in iron ore flotation - an overview. Colloid and Interface Science Communications, 2018, 25: 41-65. doi: 10.1016/j.colcom.2018.06.003
[35] LOPES G M, LIMA R. Flotao direta de minério de ferro com oleato de sódio[J]. Rem Revista Escola de Minas, 2009, 62(3): 323-329. doi: 10.1590/S0370-44672009000300010
[36] XW A, WLA B, HAO D A, et al. Potential application of an eco-friendly amine oxide collector in flotation separation of quartz from hematite[J]. Separation and Purification Technology, 2021, 278: 119668. doi: 10.1016/j.seppur.2021.119668
[37] VIDYADHAR A, KUMARI N, BHAGAT R P. Adsorption mechanism of mixed cationic/Anionic collectors in quartz-hematite flotation system[J]. Mineral Processing & Extractive Metallurgy Review, 2014, 35(2): 117-125.
[38] SAHOO H, RATH S S, RAO D S, et al. Role of silica and alumina content in the flotation of iron ores[J]. International Journal of Mineral Processing, 2016, 148: 83-91. doi: 10.1016/j.minpro.2016.01.021
[39] CRUNDWELL F K. On the mechanism of the flotation of oxides and silicates[J]. Minerals Engineering, 2016, 95: 185-196. doi: 10.1016/j.mineng.2016.06.017
[40] ZHANG P, YU Y, BOGAN M. Challenging the "Crago" double float process Ⅱ. Amine-fatty acid flotation of siliceous phosphates[J]. Minerals Engineering, 1997, 10(9): 983-994. doi: 10.1016/S0892-6875(97)00078-2
[41] 李显嵩. 湖南某低品位萤石矿浮选试验研究[J]. 非金属矿, 2011, 34(6): 36-38+41. https://www.cnki.com.cn/Article/CJFDTOTAL-FJSK201106010.htm
[42] MARTIŃEZ A L, URIBE A S. Interfacial properties of celestite and strontianite in aqueous solutions[J]. Minerals Engineering, 1995, 8(9): 1 009-1 022. doi: 10.1016/0892-6875(95)00064-W
[43] 李志勇. RA-915捕收剂在李楼铁矿选矿厂的应用[J]. 金属矿山, 2009, 11: 209-211. https://cpfd.cnki.com.cn/Article/CPFDTOTAL-YJKS200911001044.htm
[44] 林祥辉, 路平, 陈让怀, 等. 高效新品种捕收剂RA—315的制取及应用研究[J]. 矿冶工程, 1993(3): 31-35. https://www.cnki.com.cn/Article/CJFDTOTAL-KYGC199303006.htm
[45] LI Y J, LUO B B, ZHU Y M, et al. Flotation and adsorption of a new collector alpha-Bromodecanoic acid on quartz surface[J]. Minerals Engineering, 2015, 77: 86-92. doi: 10.1016/j.mineng.2015.03.003
[46] 郭玉, 寇珏, 孙体昌, 等. 十二烷基磺酸钠和月桂酸在石英表面的吸附机理研究[J]. 矿冶工程, 2015, 35(2): 50-54. doi: 10.3969/j.issn.0253-6099.2015.02.012
[47] 宋其圣, 郭新利, 苑世领, 等. 十二烷基苯磺酸钠在SiO2表面聚集的分子动力学模拟[J]. 物理化学学报, 2009, 25(6): 1 053-1 058. https://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200906005.htm
[48] 郭文达, 朱一民, 王鹏, 等. 新型酰胺基羧酸捕收剂DWD-1用于铁矿反浮选试验研究[J]. 矿产保护与利用, 2016(3): 22-25+39. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=0f0ac999-3237-46c4-acd7-2fd6a41f3b8d
[49] GUO W D, ZHU Y M, HAN Y X, et al. Flotation performance and adsorption mechanism of a new collector 2-(carbamoylamino) lauric acid on quartz surface[J]. Minerals Engineering, 2020, 153: 106343. doi: 10.1016/j.mineng.2020.106343
[50] 刘文刚. 新型赤铁矿反浮选脱硅捕收剂的合成及浮选性能研究[D]. 沈阳: 东北大学, 2010.
[51] HUANG Z, ZHONG H, WANG S, et al. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1, 2-bis(dimethyl-dodecyl-ammonium bromide)[J]. Chemical Engineering Journal, 2014, 257: 218-228. doi: 10.1016/j.cej.2014.07.057
[52] FUERSTENAU D W, JIA R. The adsorption of alkylpyridinium chlorides and their effect on the interfacial behavior of quartz[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 2004, 250(1/2/3): 223-231.
[53] ARAUJO A C, VIANA P, PERES A. Reagents in iron ores flotation[J]. Minerals Engineering, 2005, 18(2): 219-224. doi: 10.1016/j.mineng.2004.08.023
[54] 陈达, 葛英勇, 余永富. 磁选铁精矿再提纯反浮选工艺和药剂的研究[J]. 矿产保护与利用, 2005, 4: 46-50. doi: 10.3969/j.issn.1001-0076.2005.04.013
[55] CHENG Z, ZHU Y, LI Y, et al. Flotation and adsorption of quartz with the new collector butane-3-heptyloxy-1, 2-diamine[J]. Mineralogy and Petrology, 2019, 113(2): 207-216. doi: 10.1007/s00710-018-0639-y
[56] 葛英勇. 新型捕收剂烷基多胺醚(GE-609)的合成及浮选性能研究[D]. 武汉: 武汉理工大学, 2010.
[57] ABAKAWOOD G B, ADDAIMENSAH J, SKINNER W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors[J]. International Journal of Mineral Processing, 2016, 158: 55-62.
[58] VIDYADHAR A, RAO K H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system[J]. Journal of Colloid and Interface Science, 2007, 306(2): 195-204. doi: 10.1016/j.jcis.2006.10.047
[59] SWAGAT S R, HRUSHIKESH S, BISWESWAR D, et al. Density functional calculations of amines on the (101) face of quartz[J]. Minerals Engineering, 2014, 69: 57-64. doi: 10.1016/j.mineng.2014.07.007
[60] Chen P, Hu Y, Gao Z, et al. Discovery of a novel cationic surfactant: tributyltetradecyl-phosphonium chloride for iron ore flotation: from prediction to experimental verification[J]. Minerals, 2017, 7(12): 240. doi: 10.3390/min7120240
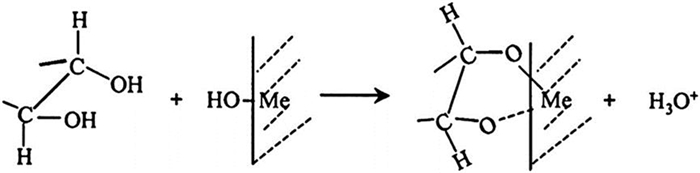
[61] JYOTI D, RATH R K, SUNATI, et al. Beneficiation of a finely disseminated low-grade iron ore by froth flotation[C]. International Seminar on Mineral Processing Technology. Allied Publishers, New Delhi, 2010.
[62] DEVíNSKY F, LACKO I, BITTEREROVA F, et al. Relationship between structure, surface activity, and micelle formation of some new bisquaternary isosteres of 1, 5-pentanediammonium dibromides[J]. Journal of Colloid & Interface Science, 1986, 114(2): 314-322.
[63] ANSARI W H, FATMA N, PANDA M, et al. Solubilization of polycyclic aromatic hydrocarbons by novel biodegradable cationic gemini surfactant ethane-1, 2-diyl bis(N, N-dimethyl-N-hexadecylammoniumacetoxy) dichloride and its binary mixtures with conventional surfactants[J]. Soft Matter, 2013, 9(5): 1 478-1 487. doi: 10.1039/c2sm26926k
[64] HUANG Z, ZHONG H, WANG S, et al. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1, 2-bis(dimethyl-dodecyl-ammonium bromide)[J]. Chemical Engineering Journal, 2014, 257: 218-228. doi: 10.1016/j.cej.2014.07.057
[65] WENG X Q, MEI G J, ZHAO T T, et al. Utilization of novel ester-containing quaternary ammonium surfactant as cationic collector for iron ore flotation[J]. Separation & Purification Technology, 2013, 103: 187-194.
[66] ZANA R. Alkanediyl-α, ω-bis(dimethylalkylammonium bromide) Surfactants: Ⅱ. Krafft Temperature and Melting Temperature[J]. Colloid Interface, 2002, 252(1): 259-261. doi: 10.1006/jcis.2002.8457
[67] 孙丽君, 吕宪俊, 杜飞飞, 等. 阳离子浮选泡沫及消泡技术研究[J]. 现代矿业, 2009, 25(6): 36-38. doi: 10.3969/j.issn.1674-6082.2009.06.012
[68] 纪斌, 孙伟, 王若林. 十二胺反浮选胶磷矿的消泡机理研究[J]. 矿冶工程, 2018, 38(2): 47-50. doi: 10.3969/j.issn.0253-6099.2018.02.011
[69] RAO D S, VIJAYAKUMAR T V, RAO S S, et al. Effectiveness of sodium silicate as gangue depressants in iron ore slimes flotation[J]. 2011, 18(5): 515-522.
[70] WANG L, SUN W, HU Y H, et al. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite - Quartz flotation system[J]. Minerals Engineering, 2014, 64: 44-50. doi: 10.1016/j.mineng.2014.03.021
[71] LIMA N P, VALADAO G, PERES A. Effect of amine and starch dosages on the reverse cationic flotation of an iron ore[J]. Minerals Engineering, 2013, 45(45): 180-184.
[72] RAO K H, ANTTI B M, FORSSBERG K. Flotation of mica minerals and selectivity between muscovite and biotite while using mixed anionic/cationic collectors[J]. Minerals & Metallurgical Processing, 2016, 7(3): 127-132.
[73] WANG Y, REN J. The flotation of quartz from iron minerals with a combined quaternary ammonium salt[J]. International Journal of Mineral Processing, 2005, 77(2): 116-122. doi: 10.1016/j.minpro.2005.03.001
[74] VIDYADHAR A, KUMARI N, BHAGAT R P. Flotation of quartz and hematite: adsorption mechanism of mixed cationic/anionic collector systems[J]. Minerals Processing, 2012, 23: 10-11.
[75] WANG Y, REN J. The flotation of quartz from iron minerals with a combined quaternary ammonium salt[J]. International Journal of Mineral Processing, 2005, 77(2): 116-122. doi: 10.1016/j.minpro.2005.03.001
[76] FILHO L S L, RODRIGUES G A. The use of ethoxylated nonionic surfactants on the cationic flotation of quartz[C]. Proceedings of the Ⅲ Meeting Southern Hemisphere on Mineral Technology, São Lourenço, Brazil, 1992: 50-64.
[77] 郭文达, 朱一民, 韩跃新, 等. 钙离子对脂肪酸类捕收剂浮选石英的影响机理[J]. 东北大学学报(自然科学版), 2018, 39(3): 409-415. https://www.cnki.com.cn/Article/CJFDTOTAL-DBDX201803021.htm
[78] COOKE S. The flotation of quartz using calcium ion activator[J]. The American Institute of Mining, Metallurgical, and Petroleum Engineers, 1949, 184: 306-309
[79] Yang H, Tang Q, Wang C, et al. Flocculation and flotation response of Rhodococcus erythropolis to pure minerals in hematite ores[J]. Minerals Engineering, 2013, 45: 67-72. doi: 10.1016/j.mineng.2013.01.005
[80] 罗溪梅, 马鸣泽, 孙传尧, 等. 铁矿石浮选体系中矿物交互影响的作用形式[J]. 中国矿业大学学报, 2018, 47(3): 645-651. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD201803019.htm
[81] SCHUHMANN R J, PRAKASH B. Effect of BaCl2 and other activators on soap flotation of quartz[J]. The American Institute of Mining, Metallurgical, and Petroleum Engineers, 1950, 187: 591-600.
[82] ZHANG J, WANG W, LIU J, et al. Fe(Ⅲ) as an activator for the flotation of spodumene, albite, and quartz minerals[J]. Minerals Engineering, 2014, 61: 16-22. doi: 10.1016/j.mineng.2014.03.004
[83] FORNASIERO D, RALSTON J. Cu(Ⅱ) and Ni(Ⅱ) activation in the flotation of quartz, lizardite and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1/2): 75-81.
[84] EJTEMAEIA M, IRANNAJADA M, GHARABAGHI M. Role of dissolved mineral species in selective flotation of smithsonite from quartz using oleate as collector[J]. International Journal of Mineral Processing, 2012, 114(8): 40-47.
[85] XINGHUA L, YONGFU Y, WEN C, et al. Effect of Selective Flocculation Desliming on Flotation of Fine Grained Yuanjiacun Iron Ore[C]. International mineral processing congress, New Delhi, India, 2012: 633.
[86] XIA L, HONG Z, LIU G, et al. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector[J]. International Journal of Mineral Processing, 2009, 92(1/2): 74-83.
[87] VIDYADHAR A, RAO K H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system[J]. Journal of Colloid and Interface Science, 2007, 306(2): 195-204. doi: 10.1016/j.jcis.2006.10.047
[88] PERES A, CORREA M I. Depression of iron oxides with corn starches[J]. Minerals Engineering, 1996, 9(12): 1 227-1 234. doi: 10.1016/S0892-6875(96)00118-5
[89] STERLING C. Textural qualities and molecular structure of starch products[J]. Texture Studies, 2007, 9(3): 225-255.
[90] NAKHAEI F, IRANNAJAD M. Reagents types in flotation of iron oxide minerals: A review[J]. Mineral Processing & Extractive Metallurgy Review, 2018, 39(2): 89-124.
[91] PAVLOVIC S, BRANDAO P. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz[J]. Minerals Engineering, 2003, 16(11): 1 117-1 122. doi: 10.1016/j.mineng.2003.06.011
[92] RATH R K, SUBRAMANIAN S, PRADEEP T. Surface Chemical Studies on Pyrite in the Presence of Polysaccharide-Based Flotation Depressants[J]. Journal of Colloid and Interface Science, 2000, 229(1): 82-91. doi: 10.1006/jcis.2000.6990
[93] QI L, ZHANG Y, LASKOWSKI J S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction[J]. International Journal of Mineral Processing, 2000, 60(3): 229-245.
[94] SHRIMALI K, MILLER J D. Polysaccharides depressants for the reverse flotation of iron ore[J]. Transactions of the Indian Institute of Metals, 2016, 69(1): 83-95. doi: 10.1007/s12666-015-0708-4
[95] SHRIMALI K, ATLURI V, YAN W, et al. The nature of hematite depression with corn starch in the reverse flotation of iron ore[J]. Journal of Colloid & Interface Science, 2018, 524: 337.
[96] ARAUJO A C, VIANA P, PERES A. Reagents in iron ores flotation[J]. Minerals Engineering, 2005, 18(2): 219-224. doi: 10.1016/j.mineng.2004.08.023
[97] RAJU G B, HOLMGREN A, FORSLING W. Adsorption of Dextrin at Mineral/Water Interface[J]. Journal of Colloid & Interface Science, 1997, 193(2): 215-222.
[98] TURRER H, PERES A. Investigation on alternative depressants for iron ore flotation[J]. Minerals Engineering, 2010, 23(11/13): 1 066-1 069.
[99] POPERECHNIKOVA O Y, FILIPPOV L O, SHUMSKAYA E N, et al. Intensification of the reverse cationic flotation of hematite ores with optimization of process and hydrodynamic parameters of flotation cell[J]. Journal of Physics Conference Series, 2017, 879: 012016. doi: 10.1088/1742-6596/879/1/012016
[100] SANTOS I, OLIVEIRA J F. Utilization of humic acid as a depressant for hematite in the reverse flotation of iron ore[J]. Minerals Engineering, 2007, 20(10): 1 003-1 007. doi: 10.1016/j.mineng.2007.03.007
[101] ENGWAYU J, PAWLIK M. Adsorption of anionic polymers on hematite - a study of zeta potential distributions[J]. Minerals Engineering, 2020, 148: 106225. doi: 10.1016/j.mineng.2020.106225
[102] TOHRY A, DEHGHAN R. Tannin: An eco-friendly depressant for the green flotation separation of hematite from quartz[J]. Minerals Engineering, 2021, 168: 106917. doi: 10.1016/j.mineng.2021.106917
-



 下载:
下载: