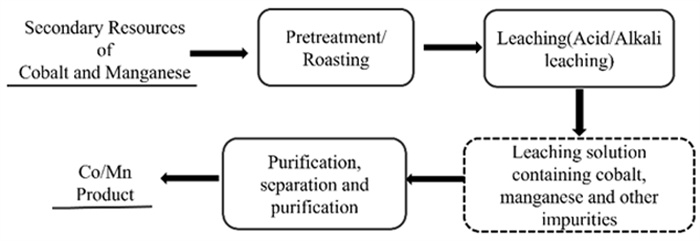
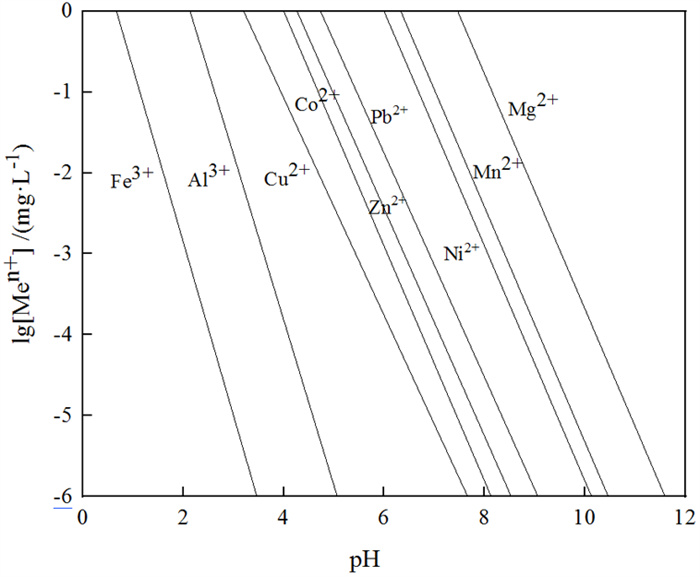
Research Progress on Separation and Extraction of Critical Metal Cobalt from Secondary Co-containing Resources
-
摘要:
当前社会对钴资源的需求量激增, 仅从矿产资源中提取钴金属已不能完全满足市场的需求。从钴的二次资源中分离提取钴是解决钴资源供应不足问题的重要途径。本文对含钴资源和废旧催化剂、钴锰废渣、废旧电池等钴锰二次资源的特点进行了分析, 对比介绍了化学沉淀法、溶剂萃取法、离子交换法、膜电解法分离溶液中钴锰离子的工艺、原理及技术效果, 同时综述了适用于溶液中钴锰分离的选冶联合方法研究进展, 其中浮游萃取法兼具界面分选与化工分离的双重优势, 钴锰分离效率高、流程短、成本低, 特别适用于选冶工业中大规模低浓度废液或废水有价金属的分离与富集, 应用前景广阔。
Abstract:At present, with the surging demand of cobalt metal, the extraction of cobalt from mineral resources can not fully meet the market requirement. Separation and extraction of cobalt from secondary resources is an important approach to relief the undersupply of cobalt. In this paper, the resource characteristics of Co mineral resources and secondary Co-containing resources, including waste catalysts, Co-Mn leaching residues, and waste batteries, were analyzed, and the processes, principles and technical effects of chemical precipitation method, solvent extraction method, ion exchange method and electrolysis method for the separation of cobalt and manganese ions from solutions were introduced and compared. In addition, the combined beneficiation and metallurgy methods which were suitable for the separation of cobalt and manganese from solutions were also reviewed. Among the combined technologies, the novel flotation-extraction method had the dual advantages of interface separation and chemical separation, and it was characterized as high separation efficiency, short process and low cost. It is especially suitable for the large-scale separation and enrichment of valuable metals in low-concentration waste liquid or wastewater in the metallurgical industry, presenting a broad application prospect.
-

-
[1] 周涛发, 范裕. 关键金属的富集机制、矿产勘查和综合利用: 前言[J]. 岩石学报, 2021, 37(9): 2599-2603. https://www.cnki.com.cn/Article/CJFDTOTAL-YSXB202109001.htm
ZHOU T F, FAN Y. Enrichment mechanism, exploration and efficient utilization of critical metal[J]. Acta Petrologica Sinica, 2021, 37(9): 2599-2603. https://www.cnki.com.cn/Article/CJFDTOTAL-YSXB202109001.htm
[2] 李文昌, 李建威, 谢桂青, 等. 中国关键矿产现状、研究内容与资源战略分析[J]. 地学前缘, 2022, 29(1): 1-13. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202201002.htm
LI W C, LI J W, XIE G Q, et al. Critical minerals in China: current status, research focus and resource strategic analysis[J]. Earth Science Frontiers, 2022, 29(1): 1-13. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202201002.htm
[3] 王永利, 徐国栋. 钴资源的开发和利用[J]. 河北北方学院学报(自然科学版), 2005(3): 24-27. https://www.cnki.com.cn/Article/CJFDTOTAL-ZJKN200503005.htm
WANG Y L, XU G D. The development and use of cobalt resource[J]. Journal of Hebei North University (Natural Science Edition), 2005(3): 24-27. https://www.cnki.com.cn/Article/CJFDTOTAL-ZJKN200503005.htm
[4] 翟明国, 吴福元, 胡瑞忠, 等. 战略性关键金属矿产资源: 现状与问题[J]. 中国科学基金, 2019, 33(2): 106-111. https://www.cnki.com.cn/Article/CJFDTOTAL-ZKJJ201902002.htm
QU M G, WU F Y, HU R Z, et al. Critical metal mineral resources: current research status and scientific issue[J]. Bulletin of National Natural Science Foundation of China, 2019, 33(2): 106-111. https://www.cnki.com.cn/Article/CJFDTOTAL-ZKJJ201902002.htm
[5] USGS. Geological survey mineral commodity summaries[EB/OL]. 2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf.
[6] 刘昱辰, 张邦胜, 刘贵清, 等. 2020年钴市场分析[J]. 中国资源综合利用, 2020, 38(11): 110-114. doi: 10.3969/j.issn.1008-9500.2020.11.030
LIU Y C, ZHANG B S, LIU G Q, et al. Market analysis of cobalt in 2020[J]. China Resources Comprehensive Utilization, 2020, 38(11): 110-114. doi: 10.3969/j.issn.1008-9500.2020.11.030
[7] 刘昱辰, 张邦胜, 刘贵清, 等. 2020年钴市场分析[J]. 中国资源综合利用, 2020, 38(11): 110-114. doi: 10.3969/j.issn.1008-9500.2020.11.030
LIU Y C, ZHANG B S, LIU G Q, et al. Market analysis of cobalt in 2020[J]. China Resources Comprehensive Utilization, 2020, 38(11): 110-114. doi: 10.3969/j.issn.1008-9500.2020.11.030
[8] 李成伟, 王家义. 全球钴资源供应现状简析[J]. 中国资源综合利用, 2018, 36(7): 102-103. doi: 10.3969/j.issn.1008-9500.2018.07.036
LI C W, WANG J Y. A brief analysis of the current status of global cobalt resource supply[J]. China Resources Comprehensive Utilization, 2018, 36(7): 102-103. doi: 10.3969/j.issn.1008-9500.2018.07.036
[9] 刘超, 陈甲斌. 全球钴资源供需形势分析[J]. 国土资源情报, 2020(10): 27-33. doi: 10.3969/j.issn.1674-3709.2020.10.005
LIU C, CHEN J B. Analysis of supply and demand situation of global cobalt resources[J]. Land and Resources Information, 2020(10): 27-33. doi: 10.3969/j.issn.1674-3709.2020.10.005
[10] 王京, 石香江, 王寿成, 等. 未来中国钴资源需求预测[J]. 中国国土资源经济, 2019, 32(10): 28-33. https://www.cnki.com.cn/Article/CJFDTOTAL-ZDKJ201910006.htm
WANG J, SHI X J, WANG S C, et al. Demand forecast of China's cobalt resource in the future[J]. Natural Resource Economics of China, 2019, 32(10): 28-33. https://www.cnki.com.cn/Article/CJFDTOTAL-ZDKJ201910006.htm
[11] 李治东, 李博慧, 辛悦. 钴锰混合催化剂催化氧化VOCs研究进展[J]. 辽宁化工, 2020, 49(12): 1529-1532. doi: 10.3969/j.issn.1004-0935.2020.12.022
LI Z D, LI B H, XIN Y. Research progress of catalytic oxidation of VOCs by cobalt-manganese mixture catalyst[J]. Liaoning Chemical Industry, 2020, 49(12): 1529-1532. doi: 10.3969/j.issn.1004-0935.2020.12.022
[12] 黄智贤, 李明明, 邱挺. PTA氧化残渣中苯甲酸和Co2+、Mn2+的分离回收[J]. 福州大学学报(自然科学版), 2020, 48(6): 800-805. https://www.cnki.com.cn/Article/CJFDTOTAL-FZDZ202006018.htm
HUANG Z X, LI M M, QIU T. Separation and recovery of benzoic acid and Co2+, Mn2+ from PTA oxidation residue[J]. Journal of Fuzhou University (Natural Science Edition), 2020, 48(6): 800-805. https://www.cnki.com.cn/Article/CJFDTOTAL-FZDZ202006018.htm
[13] 胡大锵, 杨洋. PTA废水再生处理工艺探讨[J]. 给水排水, 2014, 50(7): 43-47. doi: 10.3969/j.issn.1002-8471.2014.07.011
HU D Q, YANG Y. Probe into PTA wastewater reclamation process[J]. Water & Wastewater Engineering, 2014, 50(7): 43-47. doi: 10.3969/j.issn.1002-8471.2014.07.011
[14] 王德诚. 我国PTA生产能力继续扩大[J]. 聚酯工业, 2021, 34(1): 60. https://www.cnki.com.cn/Article/CJFDTOTAL-JZGY202101031.htm
WANG D C. PTA production capacity continues to expand in China[J]. Polyester Industry, 2021, 34(1): 60. https://www.cnki.com.cn/Article/CJFDTOTAL-JZGY202101031.htm
[15] 王玉芳, 闫丽, 王海北, 等. 复杂含钴物料处理工艺研究[J]. 矿冶, 2014, 23(2): 55-58. https://www.cnki.com.cn/Article/CJFDTOTAL-KYZZ201402013.htm
WANG Y F, YAN L, WANG H B, et al. Study on comprehensive processing of a complex cobalt sulfide material[J]. Mining & Metallurgy, 2014, 23(2): 55-58. https://www.cnki.com.cn/Article/CJFDTOTAL-KYZZ201402013.htm
[16] 林江顺, 蒋开喜. 钴锰渣除杂提钴工艺研究[J]. 有色金属, 2002(3): 36-38. https://www.cnki.com.cn/Article/CJFDTOTAL-YOUS200203010.htm
LIN J S, JIANG K X. Cobalt recovery from cobalt manganese material[J]. Nonferrous Metals, 2002(3): 36-38. https://www.cnki.com.cn/Article/CJFDTOTAL-YOUS200203010.htm
[17] 蒋闯, 周进生, 吴春明. 我国锰矿产业集群式发展的案例研究[J]. 经济纵横, 2015(9): 75-78. https://www.cnki.com.cn/Article/CJFDTOTAL-JJZH201509018.htm
JIANG C, ZHOU J S, WU C M. Case study of cluster development of Manganese industry in China[J]. Economic Review Journal, 2015(9): 75-78. https://www.cnki.com.cn/Article/CJFDTOTAL-JJZH201509018.htm
[18] 李荣念. 温和体系浸出废弃锂离子电池正极材料钴和锂的研究[D]. 徐州: 中国矿业大学, 2021.
LI R N. Research on leaching cobalt and lithium as cathode material of spent lithium-ion battery in a gentle system[D]. Xuzhou: China University of Mining and Technology, 2021.
[19] 陈燕南, 童海华. 掘金千亿退役动力电池回收赛道: 价格最高5万元一吨巨头争相入局[N]. 中国经营报, 2022-06-13(C05).
CHEN Y N, TONG H H. Denver 100 billion retired power battery recycling track: the price of up to 50, 000 yuan a ton of giants compete to enter the game[N]. China Business Journal, 2022-06-13(C05).
[20] 詹稳. 废锂离子电池中钴的回收研究[J]. 化工管理, 2019(12): 53-54. doi: 10.3969/j.issn.1008-4800.2019.12.034
ZHAN W. Recovery of cobalt from spent lithium-ion batteries[J]. Chemical Enterprise Management, 2019(12): 53-54. doi: 10.3969/j.issn.1008-4800.2019.12.034
[21] 陈玲玲, 韩俊伟, 覃文庆, 等. 铅锌冶炼渣综合利用研究进展[J]. 矿产保护与利用, 2021, 41(3): 49-55. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=37a4e951-b923-422d-9dfd-b3084b776bb7
CHEN L L, HAN J W, QIN W Q, et al. Advances in comprehensive utilization of lead-zinc smelting slag[J]. Conservation and Utilization of Mineral Resources, 2021, 41(3): 49-55. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=37a4e951-b923-422d-9dfd-b3084b776bb7
[22] 王俊杰, 谈定生, 丁家杰, 等. 湿法炼锌渣柠檬酸浸出回收钴、锌和镍[J]. 矿产保护与利用, 2021, 41(2): 137-143. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=786784f1-b390-4864-931a-d03e5c20a162
WANG J J, TAN D S, DING J J, et al. Experimental study on leaching of valuable metals from purification residue of Zinc hydrometallurgy[J]. Conservation and Utilization of Mineral Resources, 2021, 41(2): 137-143. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=786784f1-b390-4864-931a-d03e5c20a162
[23] 李明诗, 郭首义, 李浩东, 等. 废旧碱性锌锰电池综合回收钾、锌、锰[J]. 矿产保护与利用, 2020, 40(5): 134-137. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=0256c491-e9d0-4f64-94a4-2dddd8a97282
LI M S, GUO S Y, LI H D, et al. Study on comprehensive utilization of spent zinc-manganese batteries[J]. Conservation and Utilization of Mineral Resources, 2020, 40(5): 134-137. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=0256c491-e9d0-4f64-94a4-2dddd8a97282
[24] 杨晓松, 陈国强, 邵立南, 等. 有色冶金废渣处理处置技术及发展趋势[J]. 有色金属(冶炼部分), 2021(3): 31-35. doi: 10.3969/j.issn.1007-7545.2021.03.006
YANG X S, CHEN G Q, SHAO L N, et al. Disposal technology and development trend of nonferrous metallurgical waste slag[J]. Nonferrous Metals (Extractive Metallurgy), 2021(3): 31-35. doi: 10.3969/j.issn.1007-7545.2021.03.006
[25] 秦娟, 周志伟, 李超, 等. 回收PX氧化催化剂的再生研究[J]. 广东化工, 2013, 40(13): 77-78. doi: 10.3969/j.issn.1007-1865.2013.13.037
QIN J, ZHOU Z W, LI C, et al. Study on the regeneration process for recovery PX oxidation catalyst[J]. Guangdong Chemical Industry, 2013, 40(13): 77-78. doi: 10.3969/j.issn.1007-1865.2013.13.037
[26] HE H P, FENG J L, GAO X F, et al. Selective separation and recovery of lithium, nickel, MnO2, and Co2O3 from LiNi0.5Mn0.3Co0.2O2 in spent battery[J]. Chemosphere, 2022, 286(Pt 3): 131897.
[27] 何沁华. PTA生产中废钴锰催化剂资源循环利用[D]. 常州: 江苏理工学院, 2016.
HE B H. Recycling of waste cobalt manganese catalyst resources in the production of PTA[D]. Changzhou: Jiangsu University of Technology, 2016.
[28] 何显达. 人造金刚石酸洗触媒废液中镍、钴、锰回收研究[D]. 长沙: 中南大学, 2005.
HE X D. Recovery of nickel, cobalt and manganese from catalyst waste liquid of artificial diamond pickling[D]. Changsha: Central South University, 2005.
[29] KATSIAPI A, TSAKIRIDIS P E, OUSTADAKIS P, et al. Cobalt recovery from mixed Co-Mn hydroxide precipitates by ammonia-ammonium carbonate leaching[J]. Minerals Engineering, 2010, 23(8): 643-651. doi: 10.1016/j.mineng.2010.03.006
[30] 何家成. 氨法回收人造金刚石酸洗废液中的镍、钴、锰[J]. 中国物资再生, 1997(6): 10-12. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWZS199706006.htm
HE J C. Recovery of nickel, cobalt and manganese from pickling waste liquid of artificial diamond by ammonia method[J]. The China National Resources Recycling, 1997(6): 10-12. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWZS199706006.htm
[31] BARIK S P, PRABAHARAN G, KUMAR L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: laboratory and pilot scale study[J]. Journal of Cleaner Production, 2017, 147: 37-43. doi: 10.1016/j.jclepro.2017.01.095
[32] 刘爱贤, 柳云琪, 邱广敏, 等. 人造金刚石酸洗废液的回收和利用[J]. 石油大学学报(自然科学版), 1998(1): 103-105+120. https://www.cnki.com.cn/Article/CJFDTOTAL-SYDX801.029.htm
LIU A X, LIU Y Q, QIU G M, et al. Recovery and utilization of the waste acid liquid in the process of synthesizing diamond[J]. Journal of China University of Petroleum (Edition of Natural Science), 1998(1): 103-105+120. https://www.cnki.com.cn/Article/CJFDTOTAL-SYDX801.029.htm
[33] DUTTA D, KUMARI A, PANDA R, et al. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries[J]. Separation and Purification Technology, 2018, 200: 327-334. doi: 10.1016/j.seppur.2018.02.022
[34] 王艳, 周春山. 含钴废渣硫酸化焙砂浸出液中钴、铁、锰分离研究[J]. 化学世界, 2001(6): 289-290+305. doi: 10.3969/j.issn.0367-6358.2001.06.003
WANG Y, ZHOU C S. Study on the separation of cobalt, iron and manganese from the leach solution of sulphated calcined cobalt residue[J]. Chemical World, 2001(6): 289-290+305. doi: 10.3969/j.issn.0367-6358.2001.06.003
[35] BISWAL A, MAHAKUD S, BHUYAN S, et al. Recovery of Co metal and electrolytic manganese dioxide (EMD) from Co-Mn sludge[J]. Hydrometallurgy, 2015, 152: 159-168. doi: 10.1016/j.hydromet.2015.01.006
[36] NAYL A A, HAMED M M, RIZK S E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 55: 119-125.
[37] ZANTE G, BRAUN A, MASMOUDI A, et al. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents[J]. Minerals Engineering, 2020, 156.
[38] JOO S-H, SHIN S M, SHIN D, et al. Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene[J]. Hydrometallurgy, 2015, 156: 136-141.
[39] MILEVSKⅡ N A, ZINOVEVA I V, ZAKHODYAEVA Y A, et al. Separation of Li(Ⅰ), Co(Ⅱ), Ni(Ⅱ), Mn(Ⅱ), and Fe(Ⅲ) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent[J]. Hydrometallurgy, 2022, 207.
[40] WANG F, HE F, ZHAO J, et al. Extraction and separation of cobalt(Ⅱ), copper(Ⅱ) and manganese(Ⅱ) by Cyanex272, PC-88A and their mixtures[J]. Separation and Purification Technology, 2012, 93: 8-14. doi: 10.1016/j.seppur.2012.03.018
[41] ZHAO J M, SHEN X Y, DENG F L, et al. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A[J]. Separation and Purification Technology, 2011, 78(3): 345-351.
[42] PIROM T, WONGKAEW K, WANNACHOD T, et al. Separation of Co(Ⅱ) and Mn(Ⅱ) from sulphate media via a HFSLM: reaction flux model and experimental verification[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 1532-1541.
[43] 胡博, 黄凌云, 孙鑫, 等. 矿山废水处理技术研究进展[J]. 矿产保护与利用, 2021, 41(1): 46-52. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=0a948d5c-9c8c-476a-9da8-ff43f416573d
HU B, HUANG L Y, SUN X, et al. Research progress of mine wastewater treatment technology[J]. Conservation and Utilization of Mineral Resources, 2021, 41(1): 46-52. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=0a948d5c-9c8c-476a-9da8-ff43f416573d
[44] 杨海, 黄新, 林子增, 等. 离子交换法处理重金属废水的研究进展[J]. 应用化工, 2019, 48(7): 1675-1680. https://www.cnki.com.cn/Article/CJFDTOTAL-SXHG201907038.htm
YANG H, HUANG X, LIN Z Z, et al. Research progress in the treatment of heavy metal wastewater by ion exchange[J]. Applied Chemical Industry, 2019, 48(7): 1675-1680. https://www.cnki.com.cn/Article/CJFDTOTAL-SXHG201907038.htm
[45] STRAUSS M L, DIAZ L A, MCNALLY J, et al. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin[J]. Hydrometallurgy, 2021, 206.
[46] MENDES F D, MARTINS A H. Selective sorption of nickel and cobalt from sulphate solutions using chelating resins[J]. International Journal of Mineral Processing, 2004, 74(1/2/3/4): 359-371.
[47] 周杰, 宋小三, 王三反. 高浓度含铜电镀废水膜电解处理与回用[J]. 化工进展, 2021, 40(S2): 434-442. https://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2021S2052.htm
ZHOU J, SONG X S, WANG S F. Recovery and utilization of copper from electroplating wastewater with high concentration by membrane electrolysis[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 434-442. https://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2021S2052.htm
[48] LIU S P, WANG H B, JIANG K X, et al. Cobalt separation technologies and their application to scrap treatment[J]. Nonferrous Metals, 2004, 56(2): 73-76.
[49] 王成彦, 王含渊, 江培海, 等. 高锰含钴物料中钴的回收[J]. 有色金属(冶炼部分), 2005(5): 2-5. https://www.cnki.com.cn/Article/CJFDTOTAL-METE200505000.htm
WANG C Y, WANG H Y, JIANG P H, et al. Recovery of cobalt from cobalt ores with high manganese[J]. Nonferrous Metals (Mineral Processing Section), 2005(5): 2-5. https://www.cnki.com.cn/Article/CJFDTOTAL-METE200505000.htm
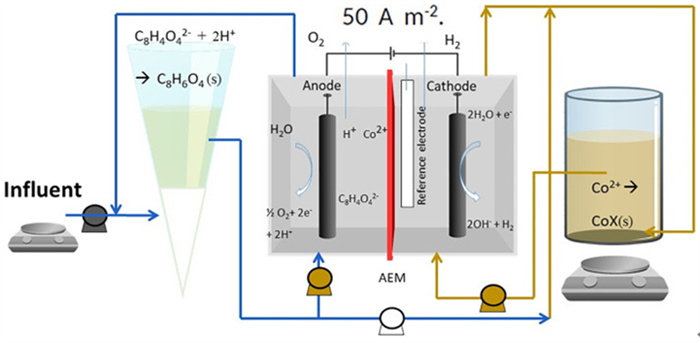
[50] GAO R, BENETTON X D, VARIA J, et al. Membrane electrolysis for separation of cobalt from terephthalic acid industrial wastewater[J]. Hydrometallurgy, 2020, 191.
[51] XING W, LEE M, CHOI S. Separation of Ag(I) by ion exchange and cementation from a raffinate containing Ag(I), Ni(Ⅱ) and Zn(Ⅱ) and traces of Cu(Ⅱ) and Sn(Ⅱ)[J]. Processes, 2018, 6(8).
[52] 柳佳建, 陈伟, 周康根, 等. 赤泥中铁的回收利用研究进展[J]. 矿产保护与利用, 2021, 41(3): 70-75. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2f07004c-acb5-4bac-b639-e2f798b8d4b0
LIU J J, CHEN W, ZHOU K G, et al. Research progress of iron recovery from red mud[J]. Conservation and Utilization of Mineral Resources, 2021, 41(3): 70-75. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2f07004c-acb5-4bac-b639-e2f798b8d4b0
[53] CHOI S, YOO K, ALORROl R D, et al. Cementation of Co ion in leach solution using Zn powder followed by magnetic separation of cementation-precipitate for recovery of unreacted Zn powder[J]. Minerals Engineering, 2020, 145.
[54] 韩桂洪, 黄艳芳, 刘兵兵, 等. 一种基于浮游萃取系统分离稀贵金属的方法: CN112538570A[P]. 2021-03-23.
HAN G H, HUANG Y F, LIU B B, et al. A method for separating rare metals based on planktonic extraction system: CN112538570A[P]. 2021-03-23.
[55] 韩桂洪, 刘兵兵, 黄艳芳, 等. 一种基于浮游萃取的溶解态高相似稀贵金属富集分离方法: CN111206150B[P]. 2022-01-28.
HAN G H, LIU B B, HUANG Y F, et al. A method for enrichment and separation of highly similar rare metals in dissolved state based on planktonic extraction: CN111206150B[P]. 2022-01-28.
[56] 韩桂洪, 刘兵兵, 黄艳芳, 等. 一种基于浮游萃取的钨钼选择性分离方法: CN111187908B[P]. 2022-01-28.
HAN G H, LIU B B, HUANG Y F, et al. A selective separation method of tungsten and molybdenum based on planktonic extraction: CN111187908B[P]. 2022-01-28.
[57] KANI O S M, AZIZITORGHABEH A, RASHCHI F. Recovery of Zn(Ⅱ), Mn(Ⅱ) and Co(Ⅱ) from the zinc plant residue using the solvent extraction with CYANEX 302 and D2EHPA/TBP: stoichiometry and structural studies[J]. Minerals Engineering, 2021, 169.
-



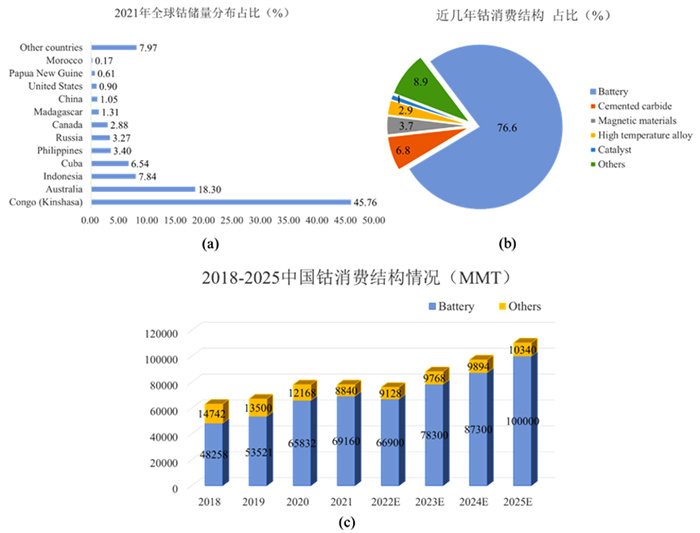
 下载:
下载: