RESEARCH PROGRESS IN BASIC CHARACTERISTICS OF GAS HYDRATE
-
摘要:
气体水合物的结构类型、热力学与动力学、界面现象与表面形貌特征等基础特性是水合物研究的重要基础,也是水合物学科发展的基石。此外,水合物在沉积物中的微观赋存形态及其对渗流的影响也是水合物的基础特性之一,是水合物储层研究的热点。笔者主要基于近3年来的文献资料,对气体水合物的结构类型、热力学与动力学、界面现象、微观赋存形态及其对渗流的影响这4个方面的研究进行了梳理与归纳,追踪学科研究前沿,较为全面地阐述了国内外气体水合物相关研究的最新进展,也提出了气体水合物基础特性研究下一步的方向与趋势,以期为多尺度、多维度研究气体水合物的基础理论与科学问题提供借鉴。
Abstract:The basic characteristics of gas hydrate, such as its structure type, thermodynamics, dynamics, interface process and surface morphology, are the major topics in gas hydrate research, which founded the cornerstone for the development of the science of natural gas hydrate. In addition, the micro-distribution of gas hydrate in sediments, as the research focus of hydrate reservoir, and its influence on gas seepage are also the basic characteristics of gas hydrate. Based on the published literatures in the past three years, the latest progress in the structural type, thermodynamics and dynamics, interface process, micro-distribution of gas hydrate and its influence on seepage characters are summarized in this paper, aiming to track the frontier of the discipline and comprehensively expounds the latest progress in the related research fields of gas hydrate both at home and abroad. Meanwhile, future direction and trend of the study on basic characteristics of gas hydrate are discussed in this paper, so as to provide a reference for multi-scale and multi-dimensional research on the basic theory of gas hydrate.
-
Key words:
- gas hydrate /
- structure type /
- thermodynamics /
- decomposition kinetics /
- surface morphology /
- micro-distribution
-

-
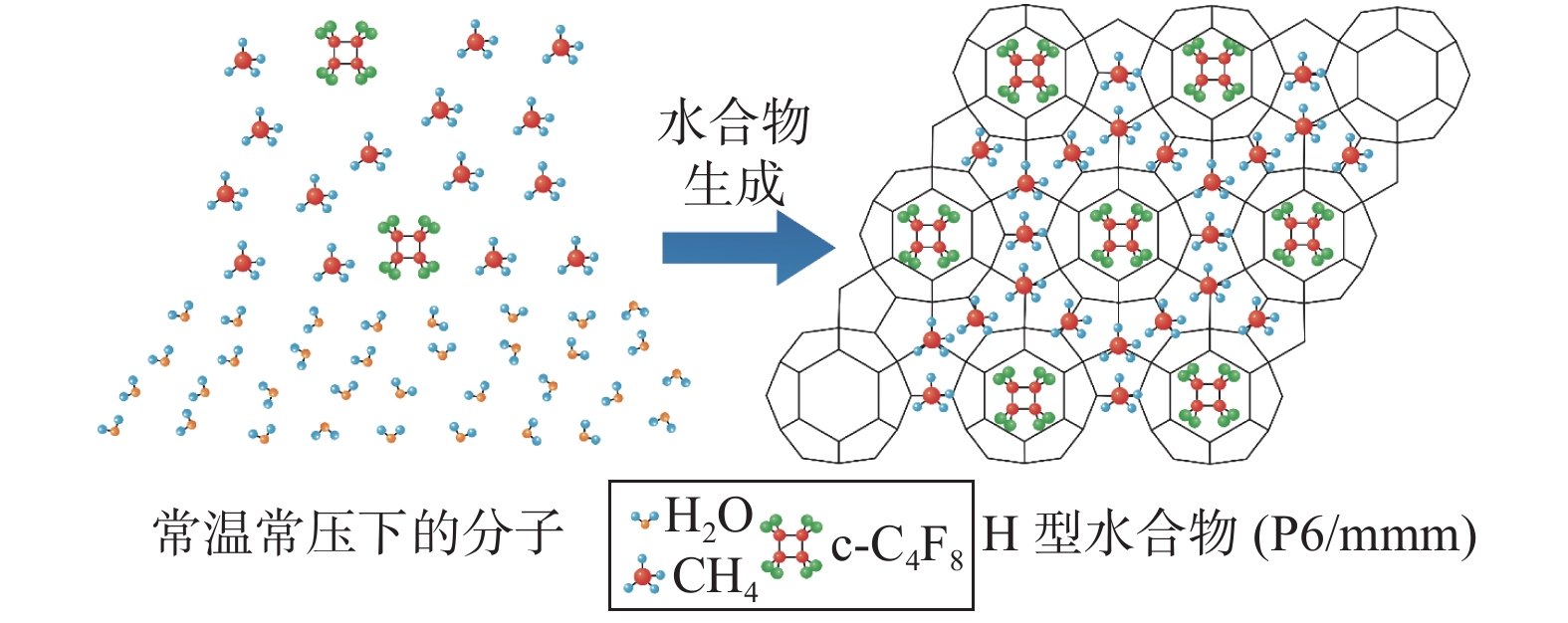
图 1 八氟环丁烷与甲烷分子形成sH型水合物示意图(据文献[3]修改)
Figure 1.
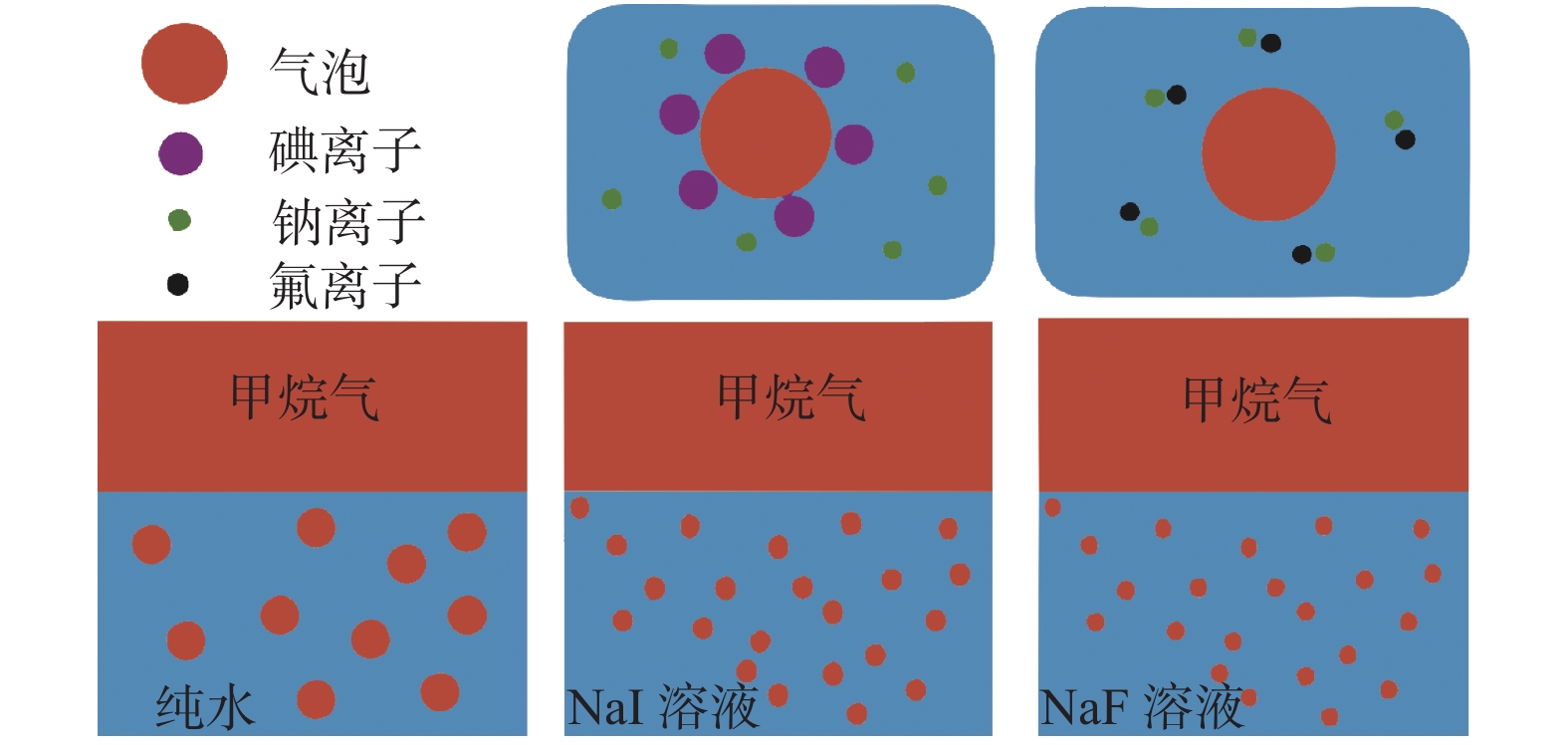
图 2 碘化钠与氟化钠离子在水溶液中甲烷气泡界面的行为(据文献[36]修改)
Figure 2.
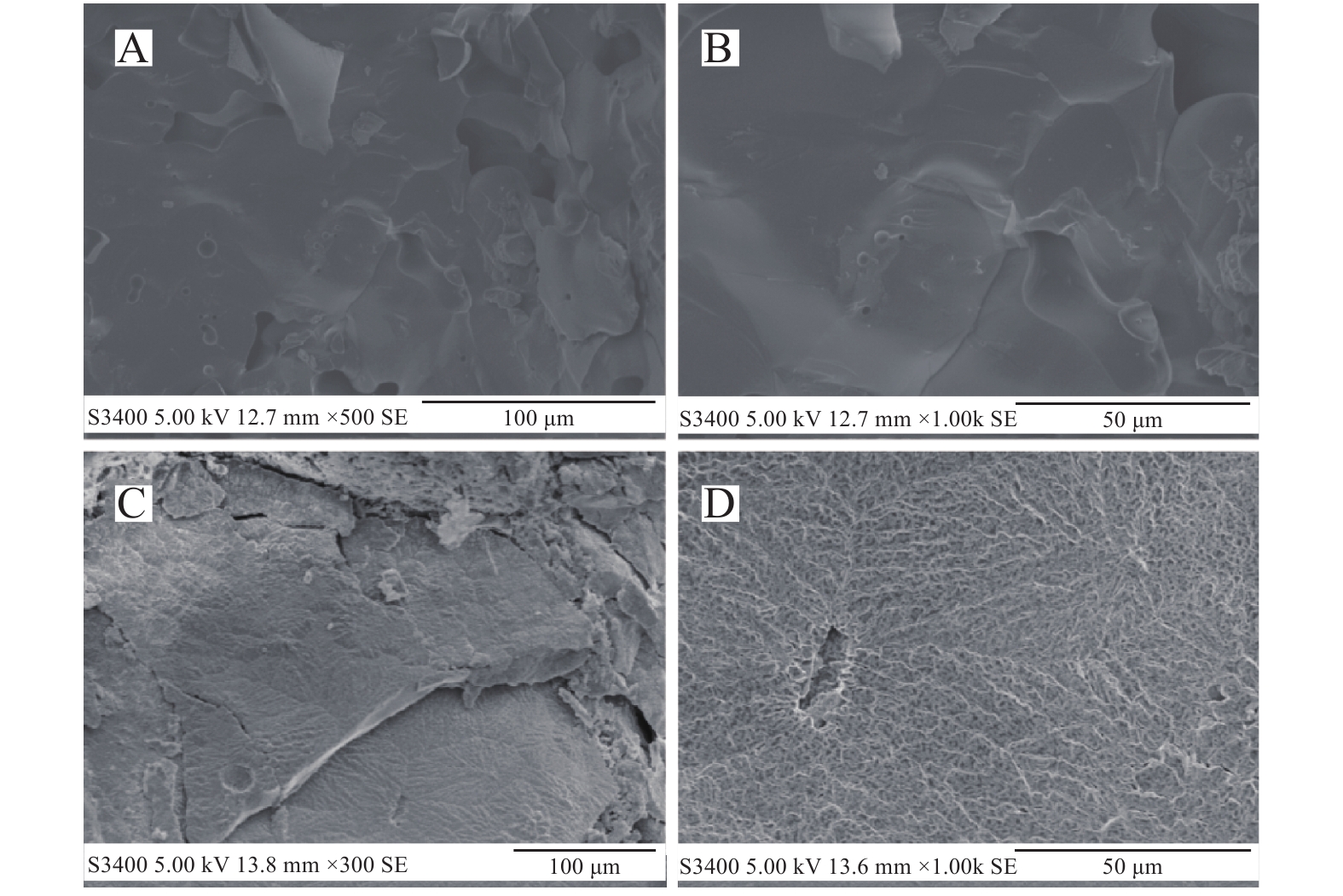
图 3 甲烷水合物(A,B)及异丁烷水合物(C,D)的表面形态(据文献[38]修改)
Figure 3.
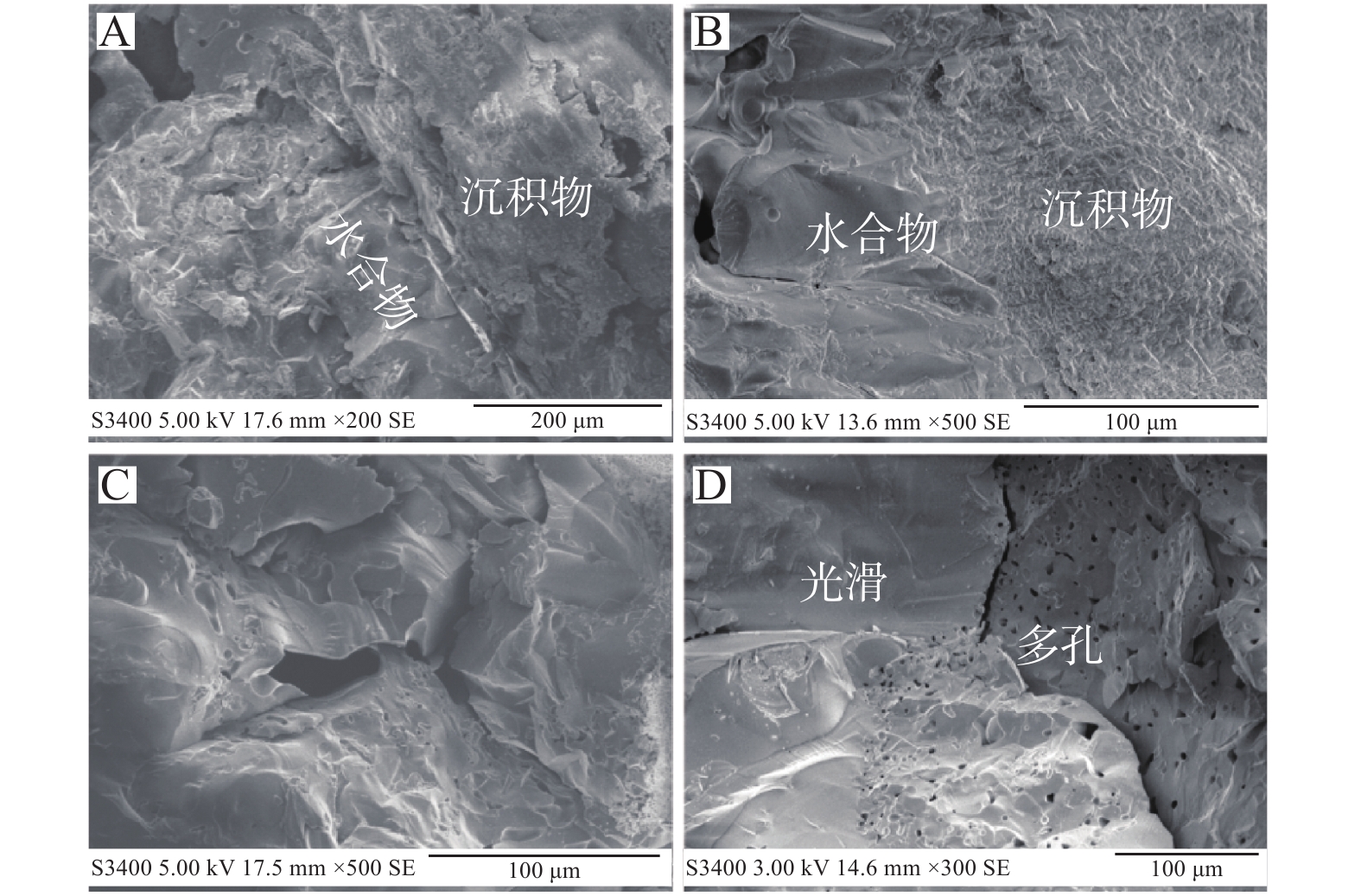
图 4 沉积物中甲烷水合物的表面形貌特征(据文献[38]修改)
Figure 4.
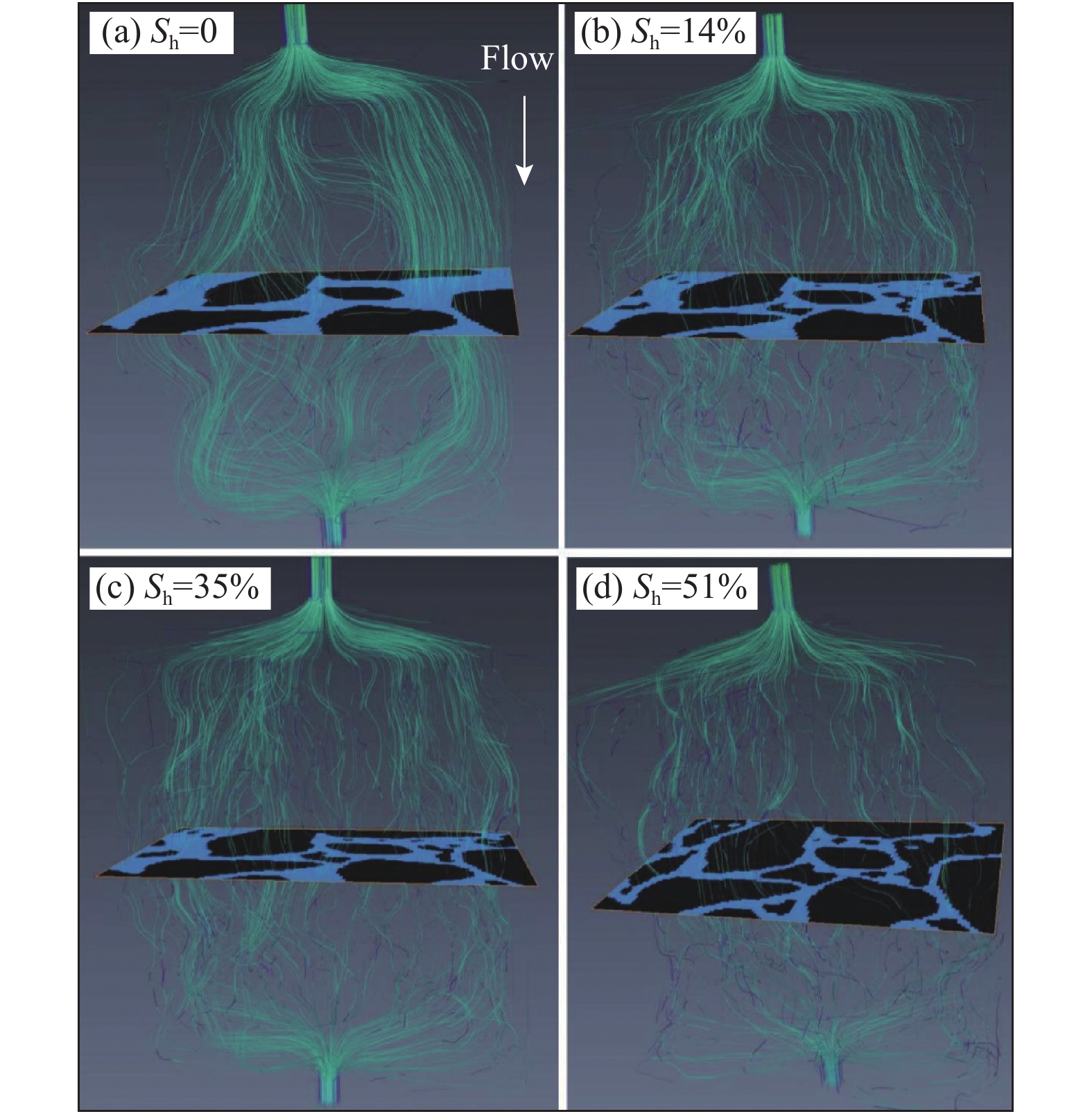
图 5 不同水合物饱和度下含水合物沉积物渗流流线图(据文献[51]修改)
Figure 5.
-
[1] Letcher M T. Future energy: Improved, sustainable and clean options for our planet (Third Edition)[M]//Boswell R, Hancock S, Yamamoto K, et al. Natural gas hydrates: Status of potential as an energy resource. Elsevier, 2020: 111-131.
[2] Sloan E D. Fundamental principles and applications of natural gas hydrate[J]. Nature,2003,426(6964):353-359. doi: 10.1038/nature02135
[3] Kim E,Seo Y. A novel discovery of a gaseous sH clathrate hydrate former[J]. Chemical Engineering Journal,2019,359:775-778. doi: 10.1016/j.cej.2018.11.170
[4] Liu C,Meng Q,Hu G,et al. Characterization of hydrate-bearing sediments recovered from the Shenhu area of the South China Sea[J]. Interpretation,2017,5(3):13-23. doi: 10.1190/INT-2016-0211.1
[5] 孟庆国,刘昌岭,李承峰,等. 常见客体分子对笼型水合物晶格常数的影响[J]. 物理化学学报,2020,1910010. doi: 10.3866/PKU.WHXB201912038
[6] Wei J,Fang Y,Lu H,et al. Distribution and characteristics of natural gas hydrates in the Shenhu Sea Area,South China Sea[J]. Marine and Petroleum Geology,2018,98:622-628. doi: 10.1016/j.marpetgeo.2018.07.028
[7] Yu Y S,Zhang Q Z,Li X S,et al. Kinetics,compositions and structures of carbon dioxide/hydrogen hydrate formation in the presence of cyclopentane[J]. Applied Energy,2020,265:114808. doi: 10.1016/j.apenergy.2020.114808
[8] Arzbacher S,Rahmatian N,Ostermann A,et al. Macroscopic defects upon decomposition of CO2 clathrate hydrate crystals[J]. Physical Chemistry Chemical Physics,2019,21(19):9694-9708. doi: 10.1039/C8CP07871H
[9] Chazallon B,Pirim C. Selectivity and CO2 capture efficiency in CO2-N2 clathrate hydrates investigated by in-situ Raman spectroscopy[J]. Chemical Engineering Journal,2018,342:171-183. doi: 10.1016/j.cej.2018.01.116
[10] Tang C,Zhou X,Li D,et al. In situ Raman investigation on mixed CH4-C3H8 hydrate dissociation in the presence of polyvinylpyrrolidone[J]. Fuel,2018,214:505-511. doi: 10.1016/j.fuel.2017.11.063
[11] Lee J,Jin Y K,Seo Y. Characterization of cyclopentane clathrates with gaseous guests for gas storage and separation[J]. Chemical Engineering Journal,2018,338:572-578. doi: 10.1016/j.cej.2018.01.054
[12] Lee Y,Choi W,Seo Y,et al. Structural transition induced by cage-dependent guest exchange in CH4+C3H8 hydrates with CO2 injection for energy recovery and CO2 sequestration[J]. Applied Energy,2018,228:229-239. doi: 10.1016/j.apenergy.2018.06.088
[13] Zhong J R,Sun Y F,Li W Z,et al. Structural transition range of methane-ethane gas hydrates during decomposition below ice point[J]. Applied Energy,2019,250(1):873-881.
[14] Zhu J,Du S,Yu X,et al. Encapsulation kinetics and dynamics of carbon monoxide in clathrate hydrate[J]. Nature Communications,2014,5:4128. doi: 10.1038/ncomms5128
[15] Petuya C,Martin-Gondre L,Aurel P,et al. Unraveling the metastability of the sI and sⅡ carbon monoxide hydrate with a combined DFT-neutron diffraction investigation[J]. The Journal of Chemical Physics,2019,150(18):184705. doi: 10.1063/1.5093202
[16] Petuya C,Damay F,Talaga D,et al. Guest partitioning in carbon monoxide by Raman spectroscopy[J]. The Journal of Physical Chemistry C,2017,121:13798-13802. doi: 10.1021/acs.jpcc.7b04947
[17] Beheshtimaal A,Haghtalab A. Thermodynamic modeling of hydrate formation conditions using different activity coefficient models in the presence of tetrahydrofuran (THF)[J]. Chemical Engineering Research and Design,2018,129:150-159. doi: 10.1016/j.cherd.2017.11.015
[18] Boufares A,Provost E,Dalmazzone D,et al. Kinetic study of CO2 hydrates crystallization:characterization using FTIR/ATR spectroscopy and contribution modeling of equilibrium/non-equilibrium phase-behavior[J]. Chemical Engineering Science,2018,192:371-379. doi: 10.1016/j.ces.2018.07.050
[19] Hu Y,Taras Y M,Prasad K,et al. Gas hydrates phase equilibria for structure I and Ⅱ hydrates with chloride salts at high salt concentra tions and up to 200 MPa[J]. The Journal of Chemical Thermodynamics,2018,117:27-32. doi: 10.1016/j.jct.2017.06.007
[20] Kim E,Ko G,Seo Y. Phase equilibria and azeotropic behavior of C2F6+N2 gas hydrates[J]. The Journal of Chemical Thermodynamics,2018,117:43-47. doi: 10.1016/j.jct.2017.06.016
[21] Dehaghani A,Karami B. A new predictive thermodynamic framework for phase behavior of gas hydrate[J]. Fuel,2018,216:796-809. doi: 10.1016/j.fuel.2017.11.128
[22] Zeng X,Wu G,Zhong J,et al. Three-scale in situ investigation on the film morphology and masstransfer channels during the thickening growth of hydrates on gas bubble[J]. Crystal Growth & Design,2019,19:3158-3165.
[23] Xu C G,Yan R,Fu J,et al. Insight into micro-mechanism of hydrate-based methane recovery and carbon dioxide capture from methane-carbon dioxide gas mixtures with thermal characterization[J]. Applied Energy,2019,239:57-69. doi: 10.1016/j.apenergy.2019.01.087
[24] Cho S J,Hai T L S,Lee J D. In-situ Raman and kinetic study on the methane hydrate formation and decomposition[J]. Energy Procedia,2019,158:5615-5621. doi: 10.1016/j.egypro.2019.01.578
[25] Zhang B,Zhou L,Liu C,et al. Influence of sediment media with different particle sizes on the nucleation of gas hydrate[J]. Natural Gas Industry B,2018,5(6):652-659. doi: 10.1016/j.ngib.2018.11.001
[26] Ke W,Svartaas T M,Chen D. A review of gas hydrate nucleation theories and growth models[J]. Journal of Natural Gas Science and Engineering,2019,61:169-196. doi: 10.1016/j.jngse.2018.10.021
[27] Casco M E,Zhang E,Grätz S,et al. Experimental evidence of confined methane hydrate in hydrophilic and hydrophobic model carbons[J]. The Journal of Physical Chemistry C,2019,123(39):24071-24079. doi: 10.1021/acs.jpcc.9b06366
[28] Thrane L W,Seymour J D,Codd S L. Probing diffusion dynamics during hydrate formation by high field NMR relaxometry and diffusometry[J]. Journal of Magnetic Resonance,2019,303:7-16. doi: 10.1016/j.jmr.2019.04.003
[29] Nakate P,Ghosh B,Das S,et al. Molecular dynamics study on growth of carbon dioxide and methane hydrate from a seed crystal[J]. Chinese Journal of Chemical Engineering,2019,27(9):2074-2080. doi: 10.1016/j.cjche.2019.02.006
[30] Zhang Y,Li Z,Deng S,et al. Molecular dynamics simulation on carbon dioxide hydrate formation[J]. Energy Procedia,2019,158:4648-4654. doi: 10.1016/j.egypro.2019.01.741
[31] Chen W,Hartman R L. Methane hydrate intrinsic dissociation kinetics measured in a microfluidic system by means of in situ raman spectroscopy[J]. Energy & fuels,2018,32(11):11761-11771.
[32] Komatsu H,Sasagawa T,Yamamoto S,et al. Methane clathrate hydrate dissociation analyzed with Raman spectroscopy and a thermodynamic mass transfer model considering cage occupancy[J]. Fluid Phase Equilibria,2019,489:41-47. doi: 10.1016/j.fluid.2019.02.004
[33] Wang B,Fan Z,Wang P,et al. Analysis of depressurization mode on gas recovery from methane hydrate deposits and the concomitant ice generation[J]. Applied Energy,2018,227:624-633. doi: 10.1016/j.apenergy.2017.09.109
[34] Kondori J,Zendehboudi S,James L. New insights into methane hydrate dissociation:Utilization of molecular dynamics strategy[J]. Fuel,2019,249:264-276. doi: 10.1016/j.fuel.2019.02.125
[35] Xu T,Lang X,Fan S,et al. The effect of electric fields in methane hydrate growth and dissociation:A molecular dynamics simulation[J]. Computational & Theoretical Chemistry,2019,1149:57-68.
[36] Asadi F,Ejtemaei M,Birkett G,et al. The link between the kinetics of gas hydrate formation and surface ion distribution in the low salt concentration regime[J]. Fuel,2019,240:309-316. doi: 10.1016/j.fuel.2018.11.146
[37] Mirzaeifard S,Servio P,Rey A D. Molecular dynamics characterization of the water-methane,ethane,and propane gas mixture interfaces[J]. Chemical Engineering Science,2019,208:114769. doi: 10.1016/j.ces.2019.01.051
[38] Sun J,Li C,Hao X,et al. Study of the surface morphology of gas hydrate[J]. Journal of Ocean University of China,2020,2:331-338.
[39] 彭 力,宁伏龙,李 维,等. 用原子力显微镜研究温度和接触界面对THF水合物形貌的影响[J]. 中国科学:物理学 力学 天文学,2019,49:034612.
[40] Huo J H,Peng Z G,Ye Z B,et al. Preparation,characterization and investigation of low hydration heat cement slurry system used in natural gas hydrate formation[J]. Journal of Petroleum Science and Engineering,2018,170:81-88. doi: 10.1016/j.petrol.2018.06.055
[41] Ma S,Sun L,Kelland M,et al. Hydrophobic hydration affects growth of clathrate hydrate:Insight from NMR relaxometric and calorimetric study[J]. Chemical Communications,2019,55(20):2936-2939. doi: 10.1039/C8CC09587F
[42] Ge X,Liu J,Fan Y,et al. Laboratory investigation into the formation and dissociation process of gas hydrate by low-field NMR technique[J]. Journal of Geophysical Research:Solid Earth,2018,123:3339-3346. doi: 10.1029/2017JB014705
[43] Ji Y K,Hou J,Cui G D,et al. Experimental study on methane hydrate formation in a partially saturated sandstone using low-field NMR technique[J]. Fuel,2019,251:82-90. doi: 10.1016/j.fuel.2019.04.021
[44] 苏俊霖,董汶鑫,冯 杰,等. 黏土表面结合水的低场核磁共振定量研究[J]. 钻井液与完井液,2018,35(1):8-12. doi: 10.3969/j.issn.1001-5620.2018.01.002
[45] Sadeq D,Iglauer S,Lebedev M,et al. Experimental pore-scale analysis of carbon dioxide hydrate in sandstone via X-Ray micro-computed tomography[J]. International Journal of Greenhouse Gas Control,2018,79:73-82. doi: 10.1016/j.ijggc.2018.10.006
[46] Lei L,Seol Y,Jarvis K. Pore-scale visualization of methane hydrate-bearing sediments with micro-CT[J]. Geophysical Research Letters,2018,45(11):5417-5426. doi: 10.1029/2018GL078507
[47] Lei L,Liu Z,Seol Y,et al. An investigation of hydrate formation in unsaturated sediments using X-ray computed tomography[J]. Journal of Geophysical Research:Solid Earth,2019,124(4):3335-3349. doi: 10.1029/2018JB016125
[48] Wang D,Wang C,Li C,et al. Effect of gas hydrate formation and decomposition on flow properties of fine-grained quartz sand sediments using X-ray CT based pore network model simulation[J]. Fuel,2018,226:516-526. doi: 10.1016/j.fuel.2018.04.042
[49] Liu T,Liu X,Zhu T. Joint analysis of P-wave velocity and resistivity for morphology identification and quantification of gas hydrate[J]. Marine and Petroleum Geology,2020,112:104036. doi: 10.1016/j.marpetgeo.2019.104036
[50] Li N,Sun Z F,Sun C Y,et al. Simulating natural hydrate formation and accumulation in sediments from dissolved methane using a large three-dimensional simulator[J]. Fuel,2018,216:612-620. doi: 10.1016/j.fuel.2017.11.112
[51] Li C,Liu C,Hu G,et al. Investigation on the multiparameter of hydrate-bearing sands using nano-focus X-ray computed tomography[J]. Journal of Geophysical Research:Solid Earth,2019,124(3):2286-2296. doi: 10.1029/2018JB015849
[52] Wang J,Zhang L,Zhao J,et al. Variations in permeability along with interfacial tension in hydrate-bearing porous media[J]. Journal of Natural Gas Science & Engineering,2018,51:141-146.
[53] Chen X,Verma R,Espinoza D N,et al. Pore-scale determination of gas relative permeability in hydrate-bearing sediments using X-ray computed micro-tomography and lattice Boltzmann method[J]. Water Resources Research,2018,54(1):600-608. doi: 10.1002/2017WR021851
[54] Liu L,Dai S,Ning F,et al. Fractal characteristics of unsaturated sands-implications to relative permeability in hydrate-bearing sediments[J]. Journal of Natural Gas Science and Engineering,2019,66:11-17. doi: 10.1016/j.jngse.2019.03.019
[55] Zhang H,Luo X,Bi J,et al. Multi-component fractal representation of multi-scale structure of natural gas hydrate-bearing sediments[J]. Journal of Natural Gas Science and Engineering,2018,60:144-152. doi: 10.1016/j.jngse.2018.10.015
[56] Kossel E,Deusner C,Bigalke N,et al. The dependence of water permeability in quartz sand on gas hydrate saturation in the pore space[J]. Journal of Geophysical Research:Solid Earth,2018,123(2):1235-1251. doi: 10.1002/2017JB014630
-



 下载:
下载: