Response characteristics of aerobic methane oxidation to oxygen concentration in marine habitats
-
摘要:
海洋生境来源的甲烷好氧氧化菌及其产生的甲烷氧化作用是否具有独特性,对氧浓度这一环境因子如何响应,目前尚不清楚。本文采用海底新鲜沉积物作为菌种来源,借助微生物培养技术,实验研究了不同氧浓度条件(0%、1%、10%和50%)下的甲烷好氧氧化过程。结果表明,完全无氧条件(0%)不能发生甲烷好氧氧化作用,实验体系的甲烷氧化速率及甲烷氧化菌总丰度随氧浓度升高而降低,当氧浓度由1%升高至50%时,甲烷氧化速率减弱了约15倍,甲烷氧化菌总丰度降低了两个数量级。甲烷氧化菌优势菌属为I型氧化菌Methylobacter属,由Methylobacter leteus和Methylobacter whittenburyi组成,氧浓度增加时Methylobacter leteus的占比随之降低,Methylobacter whittenburyi则相反。本实验中甲烷好氧氧化菌及其氧化作用的最适氧浓度条件为1%,这与采样位置的原始生存环境最为接近。在海底低氧条件叠加低温、高压等特殊生境的长期驯化下,甲烷氧化菌的最适氧浓度条件将逐渐趋于其原始生存环境。
Abstract:It is not clear whether methanotrophs and the aerobic methane oxidation of marine habitats are unique and how they respond to oxygen concentration. In this paper, experimental investigations on the aerobic oxidation of methane were conducted under different oxygen concentrations (0%、1%、10% and 50%), using fresh seabed sediments as the source of methanotrophs. The results show that aerobic methane oxidation is rejective to anoxic condition (0%). Both the oxidation rate and abundance of methanotrophs decrease as the oxygen concentration increases. When oxygen concentration increases from 1% to 50%, the oxidation rate will decrease by about 15 times, and the total abundance of methanotrophs decreases by two orders in magnitude. The dominant methanotrophs belong to type I-Methylobacter, which consist of Methylobacter leteus and Methylobacter whittenburyi. When oxygen concentration increases, the proportion of Methylobacter leteus decreases, while that of Methylobacter whittenburyi increases. The study further suggests that the optimum oxygen concentration of methanotrophs and the aerobic methane oxidation is 1%, which is very close to the original environment of the sampling location. It means that the optimum oxygen concentration of methanotrophs will gradually approach the original living environment under a long-term acclimatization in specific biotope such as that with low oxygen concentration under low temperature and high pressure.
-
Key words:
- marine habitats /
- methanotroph /
- aerobic methane oxidation /
- oxygen concentration
-

-
表 1 甲烷好氧氧化实验设计方案
Table 1. Experimental design for aerobic methane oxidation
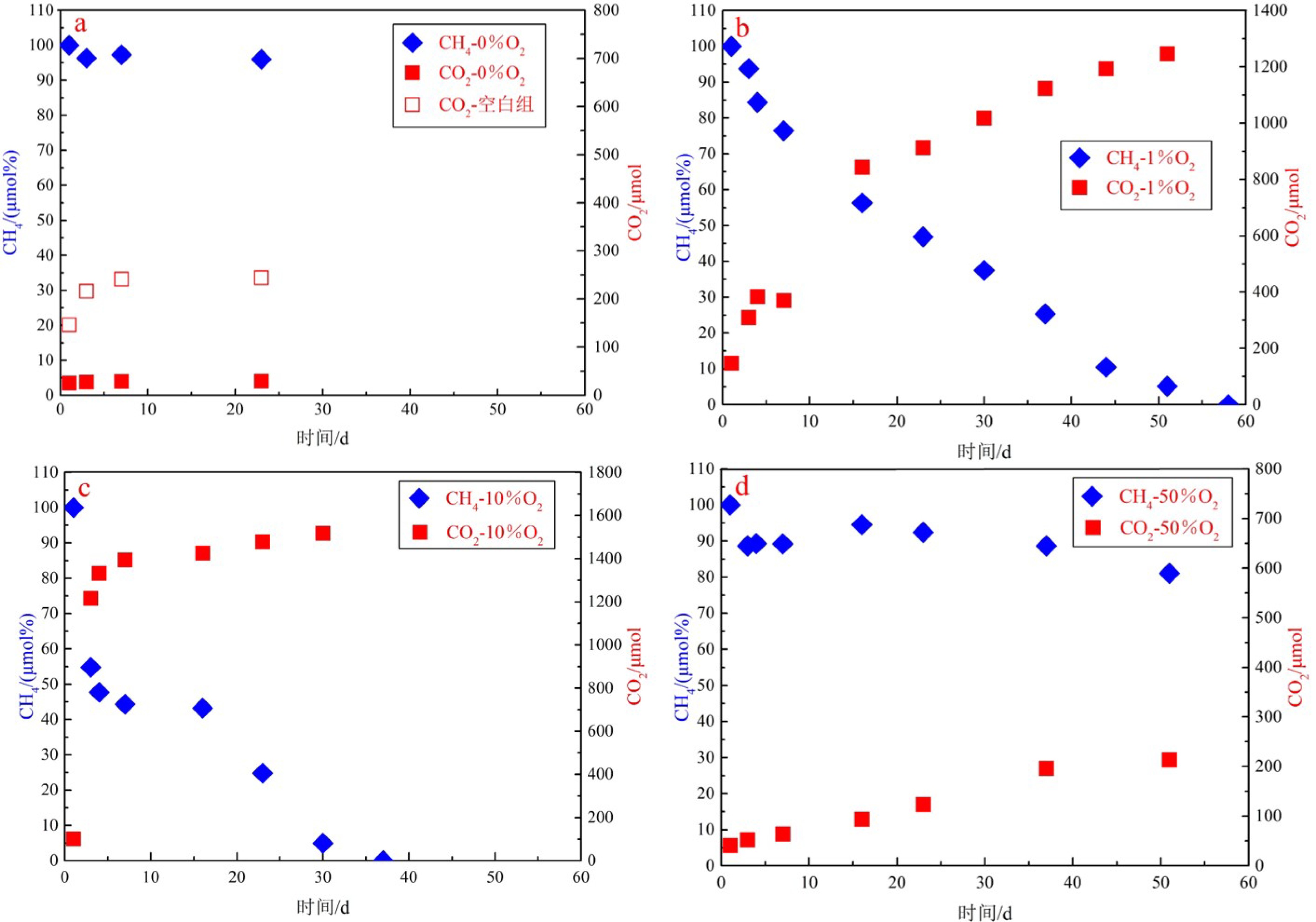
实验组 O2浓度 底物配比 气体配比 i 0%O2 11 g沉积物+30 mL海水 20 mLCH4+180 mLN2 ii 1%O2 12.7 g沉积物+30 mL海水 20 mLCH4+2 mLO2+178 mLN2 iii 10%O2 11.9 g沉积物+30 mL海水 20 mLCH4+20 mLO2+160 mLN2 iv 50%O2 12.0 g沉积物+30 mL海水 20 mLCH4+100 mLO2+80 mLN2 空白组 50%O2 11.9 g沉积物+30 mL海水 100 mLO2+100 mLN2 表 2 不同氧浓度实验过程中的甲烷氧化速率
Table 2. Methane reduction rate from each experiment at different oxygen concentrations
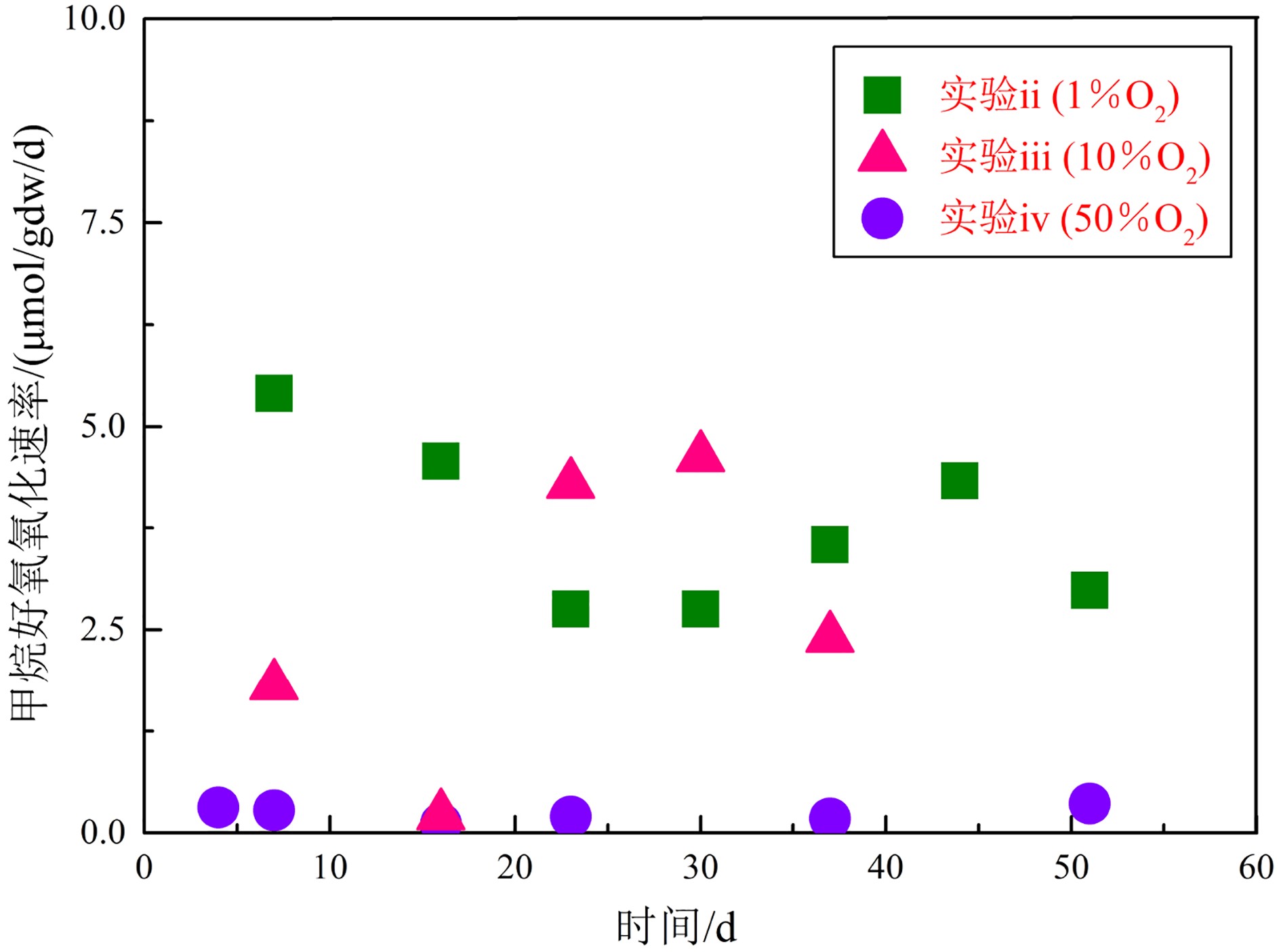
μmol/gdw/d 天数 实验ii-#1(1%O2) 实验ii-#2(1%O2) 实验iii-#1(10%O2) 实验iii-#2(10%O2) 实验iv-#1(50%O2) 实验iv-#2(50%O2) 甲烷含量快速变化阶段 1 — — — — — — 3 6.07 6.65 41.56 31.13 3.80 3.64 4 22.42 15.90 11.59 11.28 — — 甲烷含量平稳变化阶段 4 — — — — 0.35 0.28 7 3.26 7.54 1.52 2.10 0.36 0.20 16 4.40 4.75 0.18 0.24 0.08 0.15 23 3.15 2.36 3.61 4.97 0.21 0.20 30 2.36 3.15 3.96 5.28 — — 37 3.15 3.94 — 2.40 0.24 0.12 44 3.94 4.72 — — — — 51 3.01 2.96 — — 0.44 0.29 MOR平均值* 3.33 4.20 2.32 3.00 0.28 0.21 注:#1和#2分别指的是各实验中的两个平行实验组;MOR平均值*是由甲烷含量处于平稳变化阶段的气体减少速率取平均值获得。 表 3 甲烷好氧氧化菌pmoA基因α-多样性指数分析
Table 3. Analysis of α-diversity index of pmoA gene of methanotrophs
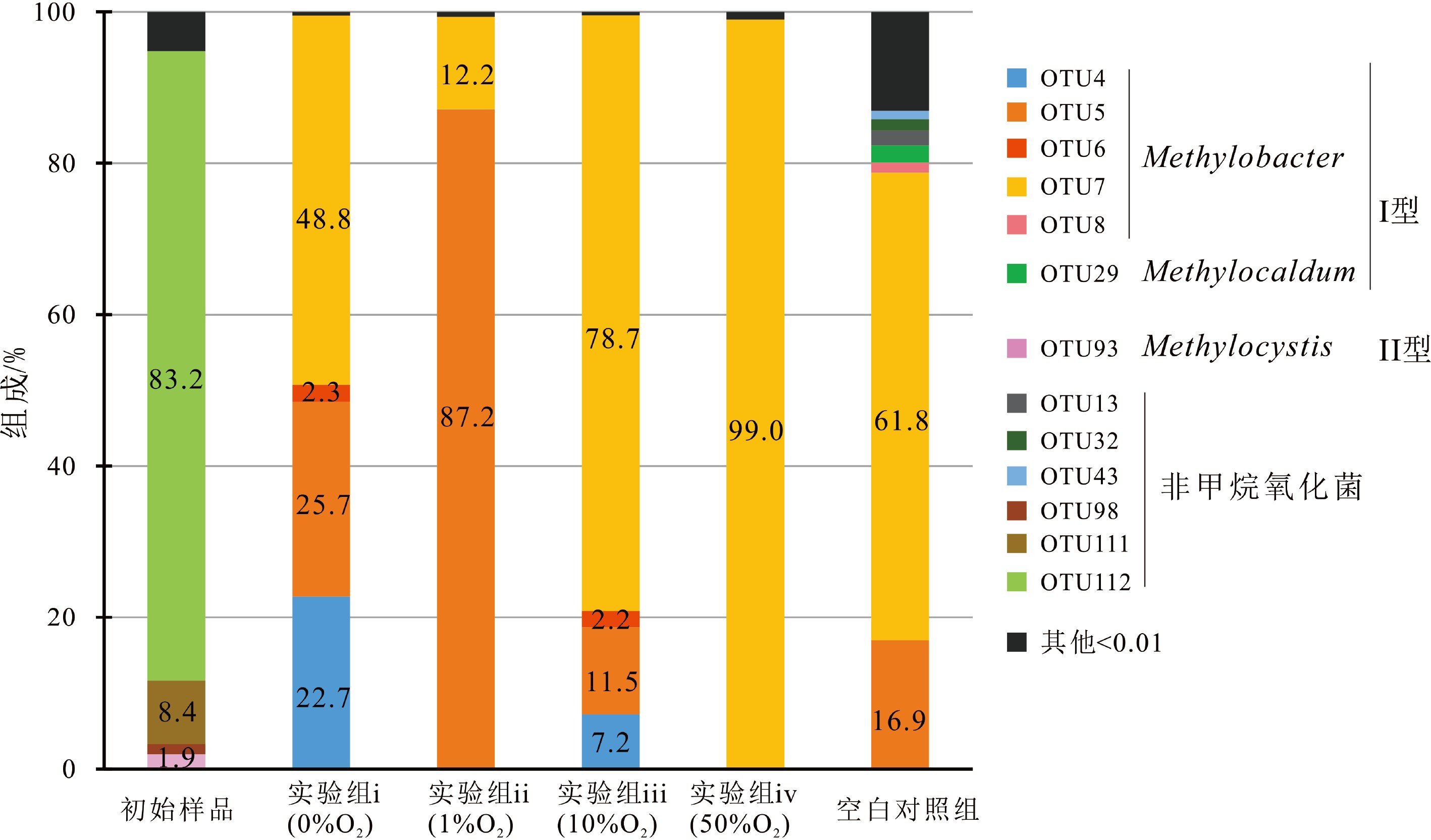
样品名称 序列/条 97%相似水平 OTUs Shannon指数 Chao 1指数 覆盖率 初始样品 14023 57 0.8 57 0.9999 实验i(0%O2) 10040 9 1.2 9 1.0000 实验ii(1%O2) 10205 5 0.4 5 1.0000 实验iii(10%O2) 22001 5 0.7 5 1.0000 实验iv(50%O2) 19694 7 0.1 7 0.9999 空白组 13730 93 1.7 93 0.9999 表 4 不同实验组的甲烷氧化菌总丰度、各菌种的绝对丰度#和平均MOR*
Table 4. Abundances of methanotrophs, each species# and average MOR*
菌群类别 OTU编号 实验i(0%O2) 实验ii(1%O2) 实验iii(10%O2) 实验iv(50%O2) 空白组 甲烷氧化菌总丰度/(copies/g) 所有OTU 3.2×105 1.9×109 2.3×108 4.1×107 1.7×105 methylobacter leteus绝对丰度/(copies/g) OTU4、5 1.5×105 1.7×109 4.4×107 3.1×105 2.9×104 methylobacter whittenburyi绝对丰度/(copies/g) OTU6、7、8 1.6×105 2.3×108 1.9×108 4.0×107 1.1×105 Methylocaldum绝对丰度/(copies/g) OTU29 0 0 0 0 3.9×103 Methylocystis绝对丰度/(copies/g) OTU93 0 0 0 0 0 平均MOR*/(μmol/gdw/d) — — 3.77 2.66 0.25 — 注:平均MOR*是由不同氧浓度实验中两个平行实验组得到的平均氧化速率;菌种的绝对丰度#=甲烷氧化菌总丰度×菌种所占百分比,百分比由高通量测序获得。 -
[1] Ul Haque M F, Xu H J, Murrell J C, et al. Facultative methanotrophs-diversity, genetics, molecular ecology and biotechnological potential: a mini-review [J]. Microbiology, 2020, 166(10): 894-908. doi: 10.1099/mic.0.000977
[2] Boetius A, Wenzhöfer F. Seafloor oxygen consumption fuelled by methane from cold seeps [J]. Nature Geoscience, 2013, 6(9): 725-734. doi: 10.1038/ngeo1926
[3] Crespo-Medina M, Meile C D, Hunter K S, et al. The rise and fall of methanotrophy following a deepwater oil-well blowout [J]. Nature Geoscience, 2014, 7(6): 423-427. doi: 10.1038/ngeo2156
[4] Leonte M, Kessler J D, Kellermann M Y, et al. Rapid rates of aerobic methane oxidation at the feather edge of gas hydrate stability in the waters of Hudson Canyon, US Atlantic Margin [J]. Geochimica et Cosmochimica Acta, 2017, 204: 375-387. doi: 10.1016/j.gca.2017.01.009
[5] Han J S, Mahanty B, Yoon S U, et al. Activity of a methanotrophic consortium isolated from landfill cover soil: response to temperature, pH, CO2, and porous adsorbent [J]. Geomicrobiology Journal, 2016, 33(10): 878-885. doi: 10.1080/01490451.2015.1123330
[6] Oshkin I Y, Belova S E, Danilova O V, et al. Methylovulum psychrotolerans sp. nov., a cold-adapted methanotroph from low-temperature terrestrial environments, and emended description of the genus Methylovulum [J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(6): 2417-2423. doi: 10.1099/ijsem.0.001046
[7] 张瑞林, 任学清. 不同压力及氧环境条件下微生物降解煤层瓦斯实验研究[J]. 煤矿安全, 2014, 45(11):1-4
ZHANG Ruilin, REN Xueqing. Experimental study on coal seam gas degradation by microorganism under different pressure and oxygen environment conditions [J]. Safety in Coal Mines, 2014, 45(11): 1-4.
[8] Li J, Liu C L, He X L, et al. Aerobic microbial oxidation of hydrocarbon gases: Implications for oil and gas exploration [J]. Marine and Petroleum Geology, 2019, 103: 76-86. doi: 10.1016/j.marpetgeo.2019.02.013
[9] Karthikeyan O P, Chidambarampadmavathy K, Nadarajan S, et al. Influence of nutrients on oxidation of low level methane by mixed methanotrophic consortia [J]. Environmental Science and Pollution Research, 2016, 23(5): 4346-4357. doi: 10.1007/s11356-016-6174-7
[10] 马若潺, 魏晓梦, 何若. 低氧生境中好氧甲烷氧化菌的缺氧耐受机理及种群结构研究进展[J]. 应用生态学报, 2017, 28(6):2047-2054
MA Ruochan, WEI Xiaomeng, HE Ruo. Mechanism of hypoxia-tolerance and community structure of aerobic methanotrophs in O2-limited environments: A review [J]. Chinese Journal of Applied Ecology, 2017, 28(6): 2047-2054.
[11] Wilshusen J H, Hettiaratchi J P A, De Visscher A, et al. Methane oxidation and formation of EPS in compost: effect of oxygen concentration [J]. Environmental Pollution, 2004, 129(2): 305-314. doi: 10.1016/j.envpol.2003.10.015
[12] Henckel T, Roslev P, Conrad R. Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil [J]. Environmental Microbiology, 2000, 2(6): 666-679. doi: 10.1046/j.1462-2920.2000.00149.x
[13] Schmidtko S, Stramma L, Visbeck M. Decline in global oceanic oxygen content during the past five decades [J]. Nature, 2017, 542(7641): 335-339. doi: 10.1038/nature21399
[14] Valentine D L, Kessler J D, Redmond M C, et al. Propane respiration jump-starts microbial response to a deep oil spill [J]. Science, 2010, 330(6001): 208-211. doi: 10.1126/science.1196830
[15] Kessler J D, Valentine D L, Redmond M C, et al. A persistent oxygen anomaly reveals the fate of spilled methane in the Deep Gulf of Mexico [J]. Science, 2011, 331(6015): 311-315.
[16] Okita N, Hoaki T, Suzuki S, et al. Characteristics of aerobic methane-oxidising bacterial community at the sea-floor surface of the Nankai Trough [J]. Marine and Freshwater Research, 2020, 71(10): 1252-1258. doi: 10.1071/MF19317
[17] Vekeman B, Dumolin C, De Vos P, et al. Improved enrichment culture technique for methane-oxidizing bacteria from marine ecosystems: the effect of adhesion material and gas composition [J]. Antonie van Leeuwenhoek, 2017, 110(2): 281-289. doi: 10.1007/s10482-016-0787-1
[18] Llanillo P J, Karstensen J, Pelegrí J L, et al. Physical and biogeochemical forcing of oxygen and nitrate changes during El Niño/El Viejo and La Niña/La Vieja upper-ocean phases in the tropical eastern South Pacific along 86° W [J]. Biogeosciences, 2013, 10(10): 6339-6355. doi: 10.5194/bg-10-6339-2013
[19] Wegener G, Boetius A. An experimental study on short-term changes in the anaerobic oxidation of methane in response to varying methane and sulfate fluxes [J]. Biogeosciences, 2009, 6(5): 867-876. doi: 10.5194/bg-6-867-2009
[20] Barbier B A, Dziduch I, Liebner S, et al. Methane-cycling communities in a permafrost-affected soil on Herschel Island, Western Canadian Arctic: active layer profiling of mcrA and pmoA genes [J]. FEMS Microbiology Ecology, 2012, 82(2): 287-302. doi: 10.1111/j.1574-6941.2012.01332.x
[21] Reim A, Lüke C, Krause S, et al. One millimetre makes the difference: high-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic–anoxic interface in a flooded paddy soil [J]. The ISME Journal, 2012, 6(11): 2128-2139. doi: 10.1038/ismej.2012.57
[22] Kalyuzhnaya M G, Yang S, Rozova O N, et al. Highly efficient methane biocatalysis revealed in a methanotrophic bacterium [J]. Nature Communications, 2013, 4(1): 2785. doi: 10.1038/ncomms3785
[23] Dannemiller K C, Lang-Yona N, Yamamoto N, et al. Combining real-time pcr and next-generation dna sequencing to provide quantitative comparisons of fungal aerosol populations [J]. Atmospheric Environment, 2014, 84: 113-121. doi: 10.1016/j.atmosenv.2013.11.036
[24] Zhang Z J, Qu Y Y, Li S Z, et al. Soil bacterial quantification approaches coupling with relative abundances reflecting the changes of taxa [J]. Scientific Reports, 2017, 7(1): 4837. doi: 10.1038/s41598-017-05260-w
[25] Bowman J P. Methylobacter[M]//Whitman W B. Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., 2015: 1-9.
[26] Bussmann I, Rahalkar M, Schink B. Cultivation of methanotrophic bacteria in opposing gradients of methane and oxygen [J]. FEMS Microbiology Ecology, 2006, 56(3): 331-344. doi: 10.1111/j.1574-6941.2006.00076.x
-



 下载:
下载: