Expansion Experiment of Multi-stage Precision Control Process for Lithium Enrichment in Qarhan Salt Lake Brine
-
摘要:
这是一篇矿业工程领域的论文。针对察尔汗盐湖老卤中锂离子的浓缩富集,采用蒸发结晶、分步分离的方法完成了老卤锂富集多级精控盐田工艺扩大实验,研究了锂、硼在蒸发过程中的富集规律,使锂离子浓度达到1 g/L以上,并获得了高品质的水氯镁石。对盐田蒸发过程进行了物料衡算,得到了各析盐阶段的实际成卤量和锂收率,以及日晒盐田的生产能力和各级盐田面积分配比例等,能够为今后实现察尔汗盐湖提钾老卤的综合开发利用提供可靠的数据支持。
Abstract:This is an article in the field of mining engineering. In view of the concentration and enrichment of lithium ions in old halide in Qerhan Salt Lake, the expansion experiment of the multi-stage precision control process of old halide enrichment was completed by means of evaporation crystallization and step separation. The enrichment law of lithium and boron during evaporation was studied. The concentration of lithium ions reached more than 1 g/L, and high quality hydrochloromagnite was obtained. The material balance of the evaporation process of the salt pan was carried out, and the actual halogen yield and lithium yield of each phase of salt extraction were obtained, as well as the production capacity of the sun salt pan and the area distribution ratio of each level of the salt pan, which can provide reliable data support for the comprehensive development and utilization of potassium old brine extraction in Qerhan Salt Lake in the future.
-
Key words:
- Mining engineering /
- Salt lake brine /
- Lithium /
- Bischofite /
- Existing state /
- Salt field process
-

-
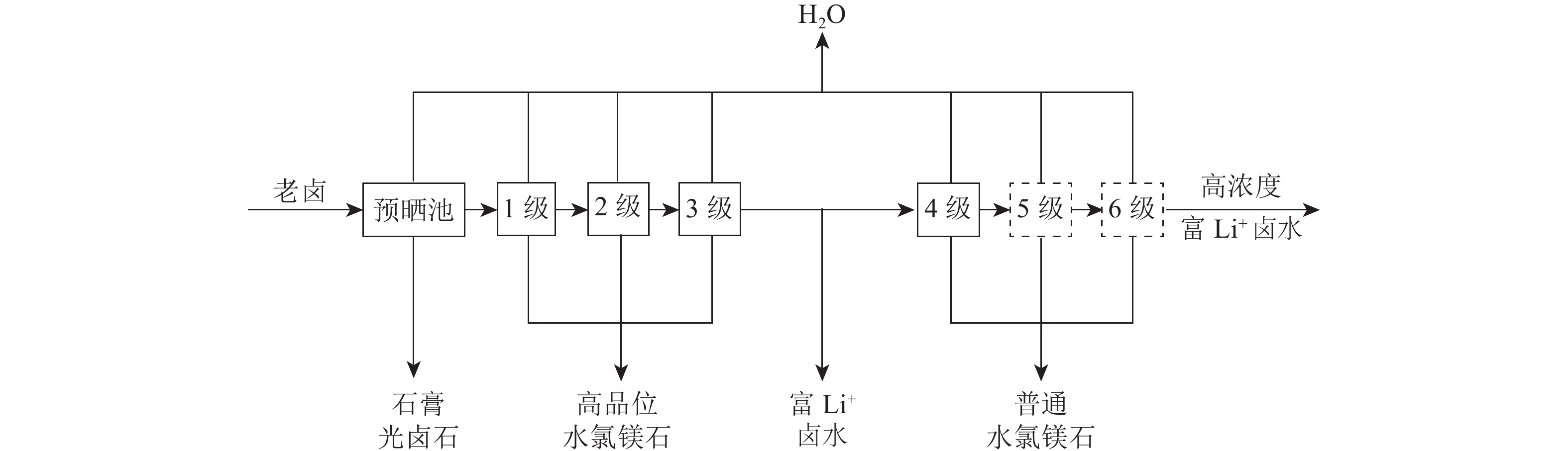
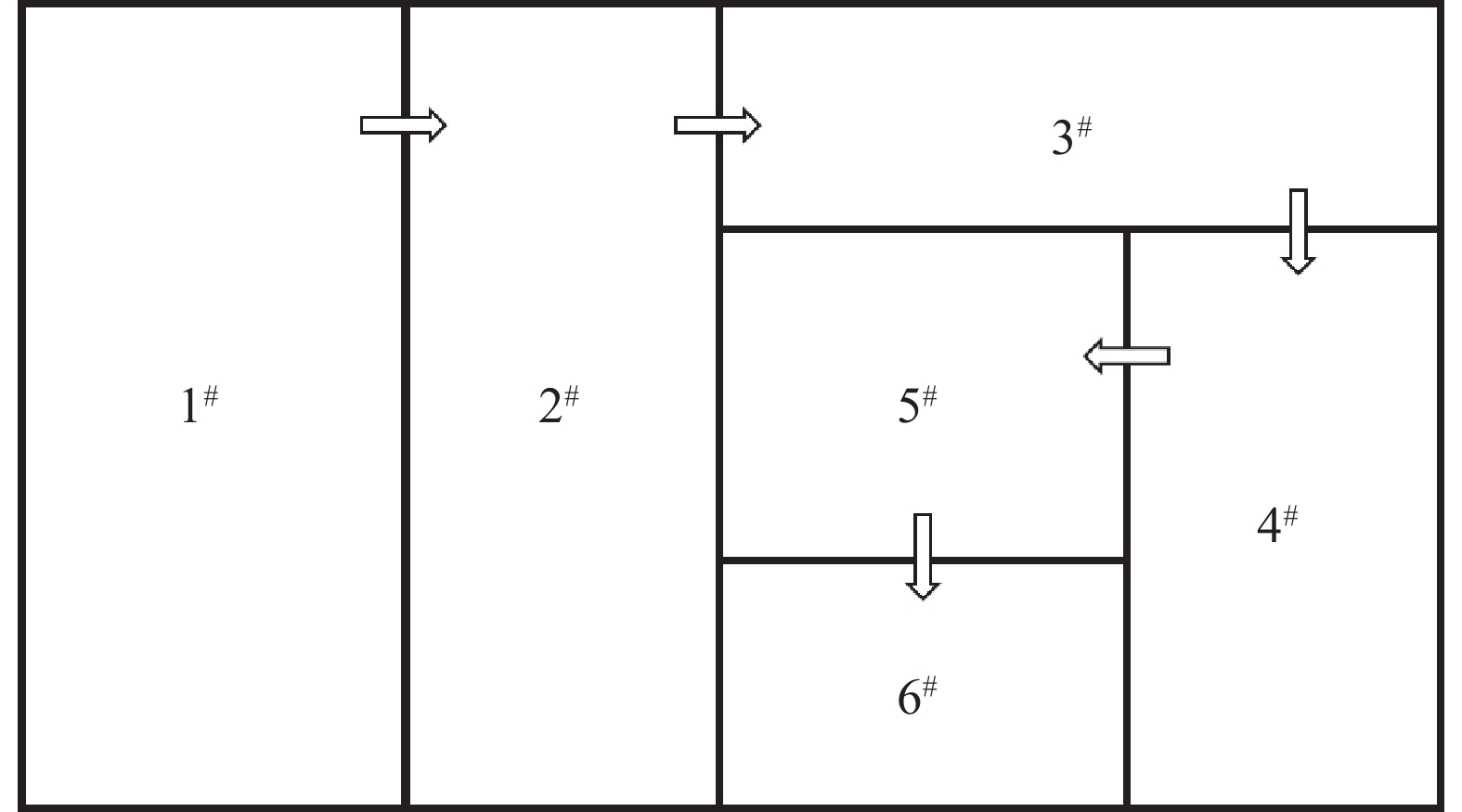
表 1 察尔汗湖区年及各月平均气象要素统计
Table 1. Statistics of annual and monthly average meteorological elements in Qarhan lake
月份 1 2 3 4 5 6 7 8 9 10 11 12 年平均值 温度/℃ -7.24 -2.2 2.8 12.8 13.9 19.0 19.7 21.5 16.8 9.1 1.1 -6.7 8.20 蒸发量/mm 41.30 74.4 166.2 224.0 373.8 392.5 541.8 467.8 285.1 149.0 84.0 36.7 2 836.70 风速/(m/s) 3.80 4.1 4.8 5.7 5.6 6.0 5.5 5.2 4.8 4.7 4.2 3.2 4.76 日照时间/h 194.40 191.5 264.2 161.3 296.9 301.7 302.0 289.9 268.4 232.2 193.2 189.1 3 358.00 相对湿度/% 44.90 36.0 32.4 30.1 28.6 36.5 33.6 28.6 27.3 33.4 33.8 40.2 33.90 表 2 盐田蒸发实验老卤化学组成
Table 2. Chemical composition of old halogenation in evaporation test of salt pan
Mg2+/% Cl-/% SO42-/% Li+/% B/% Li+/( mg/L) B/ (mg/L) 8.34 24.85 0.020 1 0.014 2 0.019 5 188.27 259.04 表 3 各级盐田物料配比及面积
Table 3. Salt pan material ratio and area at all levels
进口卤
水量/t出口卤
水量/t进卤Li+
浓度/(mg/L)出卤Li+
浓度/(mg/L)盐田面积
百分率/%理论
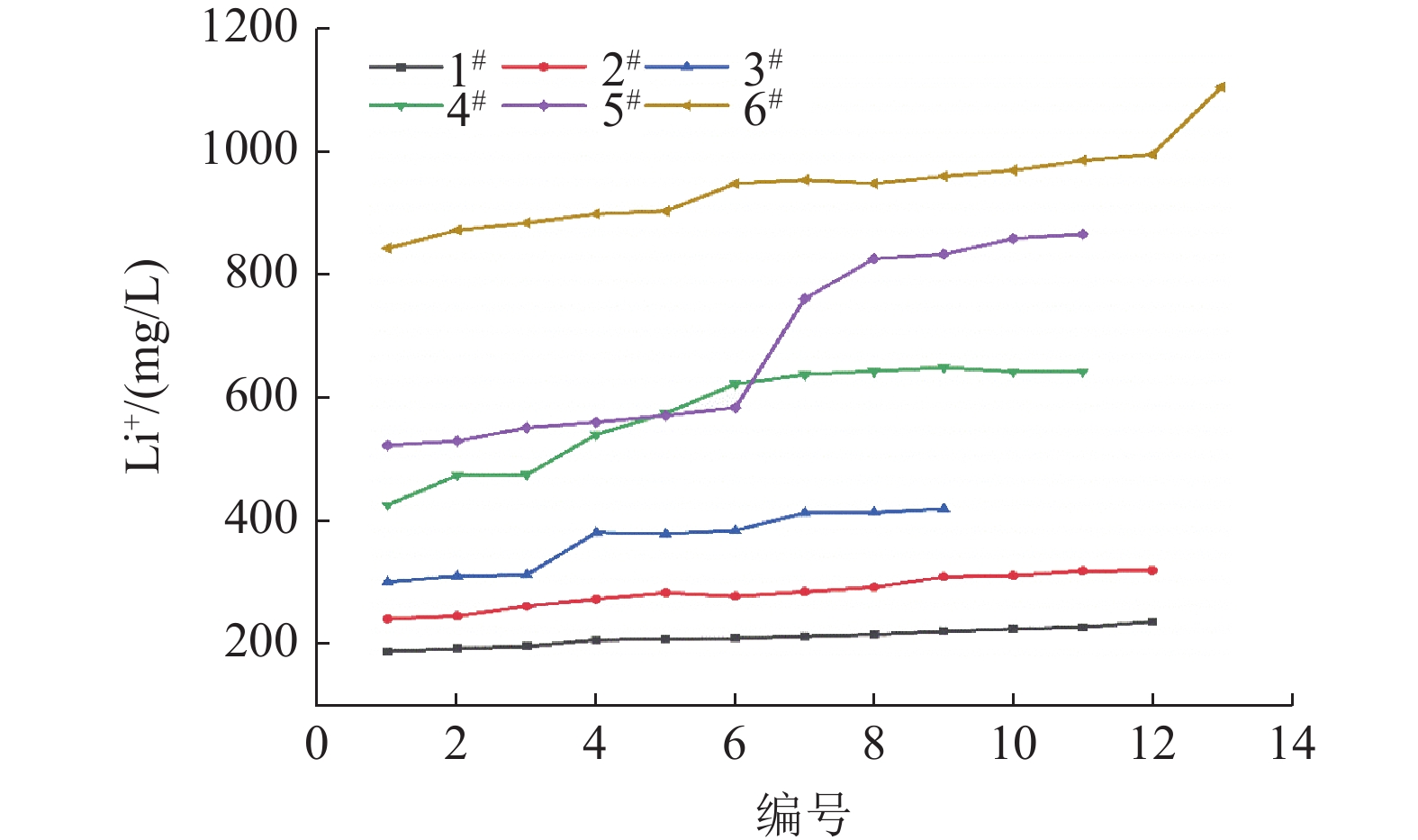
面积/m21级 100.00 68.47 180.0 241.2 35.2 1 889.2 2级 68.47 46.88 241.2 323.2 23.9 1 284.9 3级 46.88 32.10 323.2 433.1 16.5 885.6 4级 32.10 21.98 433.1 580.3 11.3 606.5 5级 21.98 15.05 580.3 777.6 7.8 418.6 6级 15.05 10.31 777.6 1 042.0 5.3 284.4 表 4 盐田蒸发实验各分级点Li+浓度/(mg/L)
Table 4. Li+ concentration at each stage of evaporation test in salt pans
原卤 1# 2# 3# 4# 5# 6# 计算分级控制点 188.27 250.40 333.03 442.93 589.10 783.50 1 042.05 实验分级控制点 188.27 236.15 319.50 419.75 642.75 865.75 1 105.00 表 5 盐田水氯镁石矿组成/%
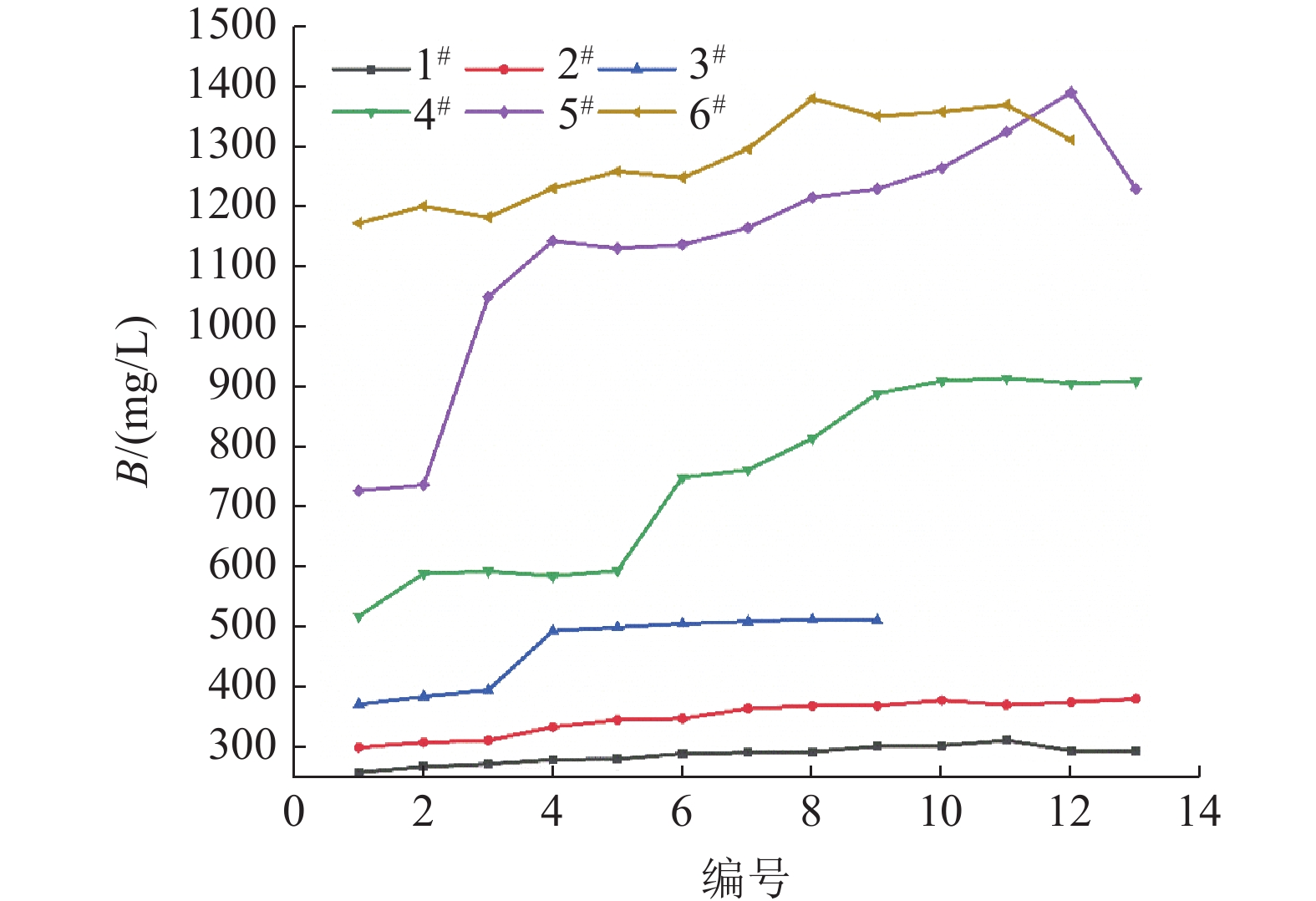
Table 5. Composition of bischofite in salt pans
名称 Mg2+ Cl- SO42- B(l) 干基MgCl2 1#盐田 11.38 33.69 0.0144 6.5×10-4 45.13 2#盐田 11.33 33.78 0.0269 2.3×10-3 44.93 3#盐田 11.45 34.19 0.0079 4.0×10-3 45.55 4#盐田 11.29 33.59 0.0341 4.6×10-3 45.05 5#盐田 11.44 33.45 0.0253 8.7×10-3 45.41 6#盐田 11.41 33.39 0.0152 1.2×10-2 45.25 平均 11.38 33.68 0.0206 5.37×-10-3 45.22 表 6 各级盐田水氯镁石矿离子含量/%
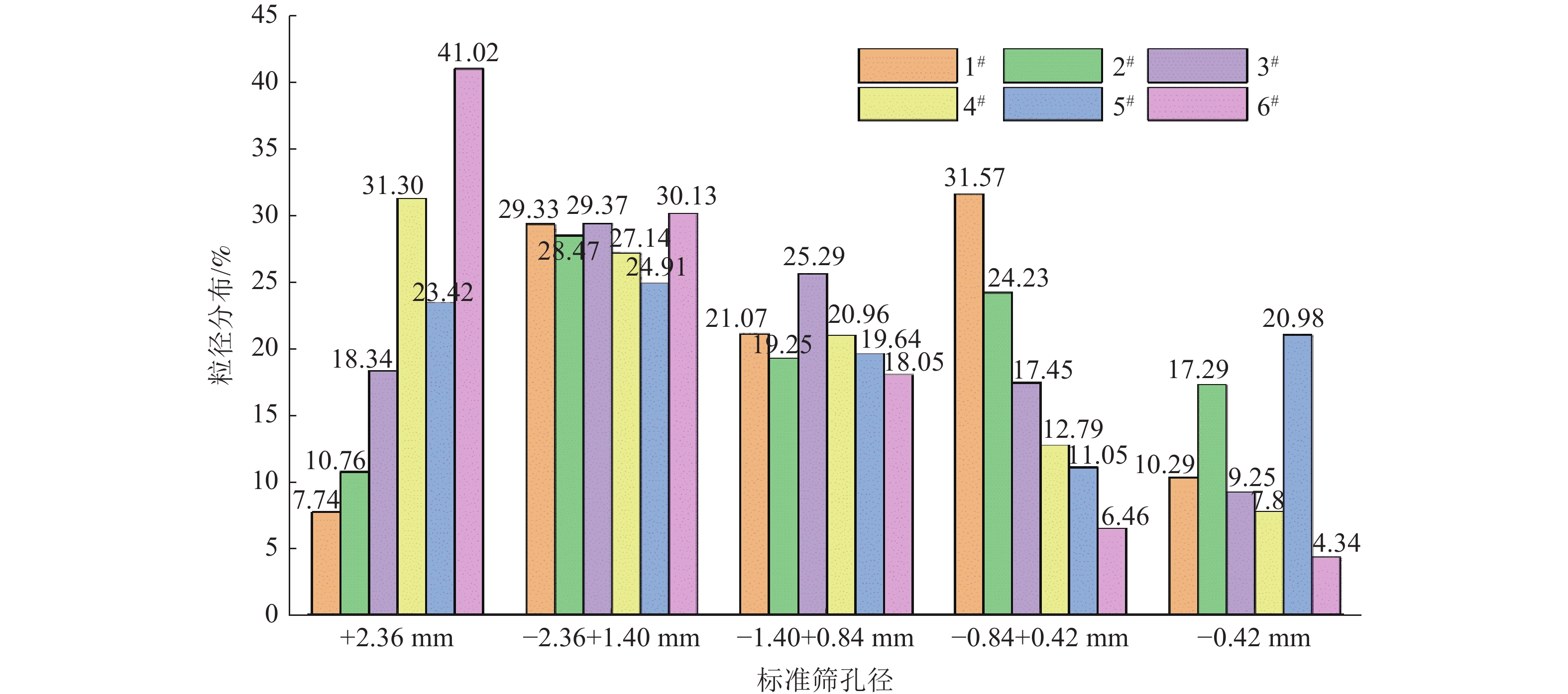
Table 6. Composition of bischofite in different levels of salt pans
粒径/mm 1# 2# 3# Mg2+ Cl- SO42- B Mg2+ Cl- SO42- B Mg2+ Cl- SO42- B +2.36 11.19 33.58 0.0124 0.0026 11.29 33.20 0.0182 0.0030 11.24 33.61 0.0083 0.0053 -2.36+1.40 11.46 33.73 0.0096 0.0009 11.32 33.57 0.0166 0.0036 11.50 33.81 0.0045 0.0041 -1.40+0.84 11.43 34.06 0.0068 0.0002 11.53 34.74 0.0106 0.0015 11.57 34.62 0.0020 0.0036 -0.84+0.42 11.38 33.54 0.0072 0.0002 11.47 33.89 0.0053 0.0021 11.61 34.35 0.0000 0.0033 -0.42 11.45 33.55 0.0669 0.0008 11.06 33.48 0.0974 0.0009 11.34 34.54 0.0491 0.0031 平均值 11.38 33.69 0.0206 0.0009 11.33 33.78 0.0296 0.0022 11.45 34.19 0.0128 0.0039 粒径/mm 4# 5# 6# Mg2+ Cl- SO42- B Mg2+ Cl- SO42- B Mg2+ Cl- SO42- B +2.36 10.99 32.11 0.0270 0.0074 11.45 32.94 0.0442 0.0175 11.16 32.31 0.0168 0.0163 -2.36+1.40 11.17 33.35 0.0206 0.0056 11.48 33.26 0.0322 0.0120 11.48 33.27 0.0143 0.0106 -1.40+0.84 11.53 34.62 0.0307 0.0024 11.55 33.55 0.0236 0.0049 11.50 33.94 0.0152 0.0082 -0.84+0.42 11.53 33.95 0.0144 0.0014 11.49 33.91 0.0169 0.0022 11.69 33.68 0.0120 0.0064 -0.42 mm 11.22 33.91 0.1509 0.0013 11.24 33.58 0.0021 0.0021 11.21 33.73 0.0119 0.0105 平均值 11.29 33.59 0.0487 0.0036 11.44 33.45 0.0238 0.0077 11.41 33.87 0.0140 0.0140 表 7 盐田日晒蒸发过程中物料衡算
Table 7. Material balance in evaporation process of salt pan
密度/(g/cm3) Li+/(mg/L) B/(mg/L) 进卤量/t Li+浓缩倍数 成矿量/t 出卤量/t Li+回收率/% 原料卤水 1.3243 188.27 259.04 100.00 1# 1.3393 236.15 294.75 1601.0 1.25 300.1 1220.0 94.73 2# 1.3479 319.50 377.50 1220.0 1.35 296.5 844.4 88.11 3# 1.3399 419.75 511.75 864.7 1.31 187.3 607.1 83.73 4# 1.3481 642.75 910.00 629.4 1.53 197.2 357.4 75.01 5# 1.3392 865.75 1017.50 387.7 1.35 86.9 247.4 70.40 6# 1.3505 1105.00 1311.50 265.8 1.28 50.74 183.1 65.97 -
[1] 张苏江, 张琳, 姜爱玲, 等. 中国盐湖资源开发利用现状与发展建议[J]. 无机盐工业, 2022, 54(10):13-21.ZHANG S J, ZHANG L, JIANG A L, et al. Current situation and development suggestions of development and utilization of salt lake resources in China[J]. Inorganic Chemicals Industry, 2022, 54(10):13-21.
ZHANG S J, ZHANG L, JIANG A L, et al. Current situation and development suggestions of development and utilization of salt lake resources in China[J]. Inorganic Chemicals Industry, 2022, 54(10):13-21.
[2] 熊增华, 王石军, 薛红魁. 察尔汗镁资源开发SWOT分析及发展构想[J]. 矿产综合利用, 2021(5):45-51.XIONG Z H, WANG S J, XUE H K. SWOT analysis and development conception of magnesium resources development in Qarhan[J]. Multipurpose Utilization of Mineral Resources, 2021(5):45-51. doi: 10.3969/j.issn.1000-6532.2021.05.007
XIONG Z H, WANG S J, XUE H K. SWOT analysis and development conception of magnesium resources development in Qarhan[J]. Multipurpose Utilization of Mineral Resources, 2021(5):45-51. doi: 10.3969/j.issn.1000-6532.2021.05.007
[3] 李燕, 王敏, 赵有璟, 等. 盐湖卤水锂资源提取技术及开发现状[J]. 盐湖研究, 2023, 31(2):71-80.LI Y, WANG M, ZHAO Y J, et al. Technology and development of lithium extraction from salt lake brine[J]. Journal of Salt Lake Research, 2023, 31(2):71-80. doi: 10.12119/j.yhyj.202302010
LI Y, WANG M, ZHAO Y J, et al. Technology and development of lithium extraction from salt lake brine[J]. Journal of Salt Lake Research, 2023, 31(2):71-80. doi: 10.12119/j.yhyj.202302010
[4] 熊增华, 王兴富, 王石军, 等. 青海盐湖锂资源综合利用规模探讨[J]. 盐湖研究, 2020, 28(4):125-131.XIONG Z H, WANG X F, WANG S J, et al. Discussion on comprehensive utilization scale of lithium resources in Qinghai salt lakes[J]. Journal of Salt Lake Research, 2020, 28(4):125-131.
XIONG Z H, WANG X F, WANG S J, et al. Discussion on comprehensive utilization scale of lithium resources in Qinghai salt lakes[J]. Journal of Salt Lake Research, 2020, 28(4):125-131.
[5] 徐正震, 梁精龙, 李慧, 等. 含锂资源中锂的提取研究现状及展望[J]. 矿产综合利用, 2021(5):32-37.XU Z Z, LIANG J L, LI H, et al. Research status and prospects of lithium extraction from lithium containing resources[J]. Multipurpose Utilization of Mineral Resources, 2021(5):32-37. doi: 10.3969/j.issn.1000-6532.2021.05.005
XU Z Z, LIANG J L, LI H, et al. Research status and prospects of lithium extraction from lithium containing resources[J]. Multipurpose Utilization of Mineral Resources, 2021(5):32-37. doi: 10.3969/j.issn.1000-6532.2021.05.005
[6] 杨小东, 赵宏彦. 内陆盐湖滩晒盐田的设计与应用[J]. 四川建材, 2021, 47(6):240-241.YANG X D, ZHAO H Y. Design and application of inland salt lake beach salt field[J]. Sichuan Building Materials, 2021, 47(6):240-241.
YANG X D, ZHAO H Y. Design and application of inland salt lake beach salt field[J]. Sichuan Building Materials, 2021, 47(6):240-241.
[7] 王冀洺, 乜贞, 樊馥, 等. 西台吉乃尔盐湖盐田与模拟蒸发过程卤水钾、锂的损失研究[J]. 无机盐工业, 2023, 55(5):31-38.WANG J M, NIE Z, FAN F, et al. Study on potassium and lithium resource loss in brine of west taijinar salt lake and simulated evaporation process[J]. Inorganic Chemicals Industry, 2023, 55(5):31-38.
WANG J M, NIE Z, FAN F, et al. Study on potassium and lithium resource loss in brine of west taijinar salt lake and simulated evaporation process[J]. Inorganic Chemicals Industry, 2023, 55(5):31-38.
[8] 陈文祥. 盐田工艺控制管理系统的初探与研究[J]. 盐科学与化工, 2021, 50(7):29-32.CHEN W X. Preliminary exploration and research on process control and management system of salt field[J]. Journal of Salt Science and Chemical Industry, 2021, 50(7):29-32. doi: 10.3969/j.issn.2096-3408.2021.07.009
CHEN W X. Preliminary exploration and research on process control and management system of salt field[J]. Journal of Salt Science and Chemical Industry, 2021, 50(7):29-32. doi: 10.3969/j.issn.2096-3408.2021.07.009
[9] 乜贞, 伍倩, 丁涛, 等. 中国盐湖卤水提锂产业化技术研究进展[J]. 无机盐工业, 2022, 54(10):1-12.NIE Z, WU Q, DING T, et al. Research progress on industrialization technology of lithium extraction from salt lake brine in China[J]. Inorganic Chemicals Industry, 2022, 54(10):1-12.
NIE Z, WU Q, DING T, et al. Research progress on industrialization technology of lithium extraction from salt lake brine in China[J]. Inorganic Chemicals Industry, 2022, 54(10):1-12.
[10] 王琪, 赵有璟, 刘洋, 等. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6):2905-2921+3433.WANG Q, ZHAO Y J, LIU Y, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J]. CIESC Journal, 2021, 72(6):2905-2921+3433. doi: 10.11949/0438-1157.20201715
WANG Q, ZHAO Y J, LIU Y, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J]. CIESC Journal, 2021, 72(6):2905-2921+3433. doi: 10.11949/0438-1157.20201715
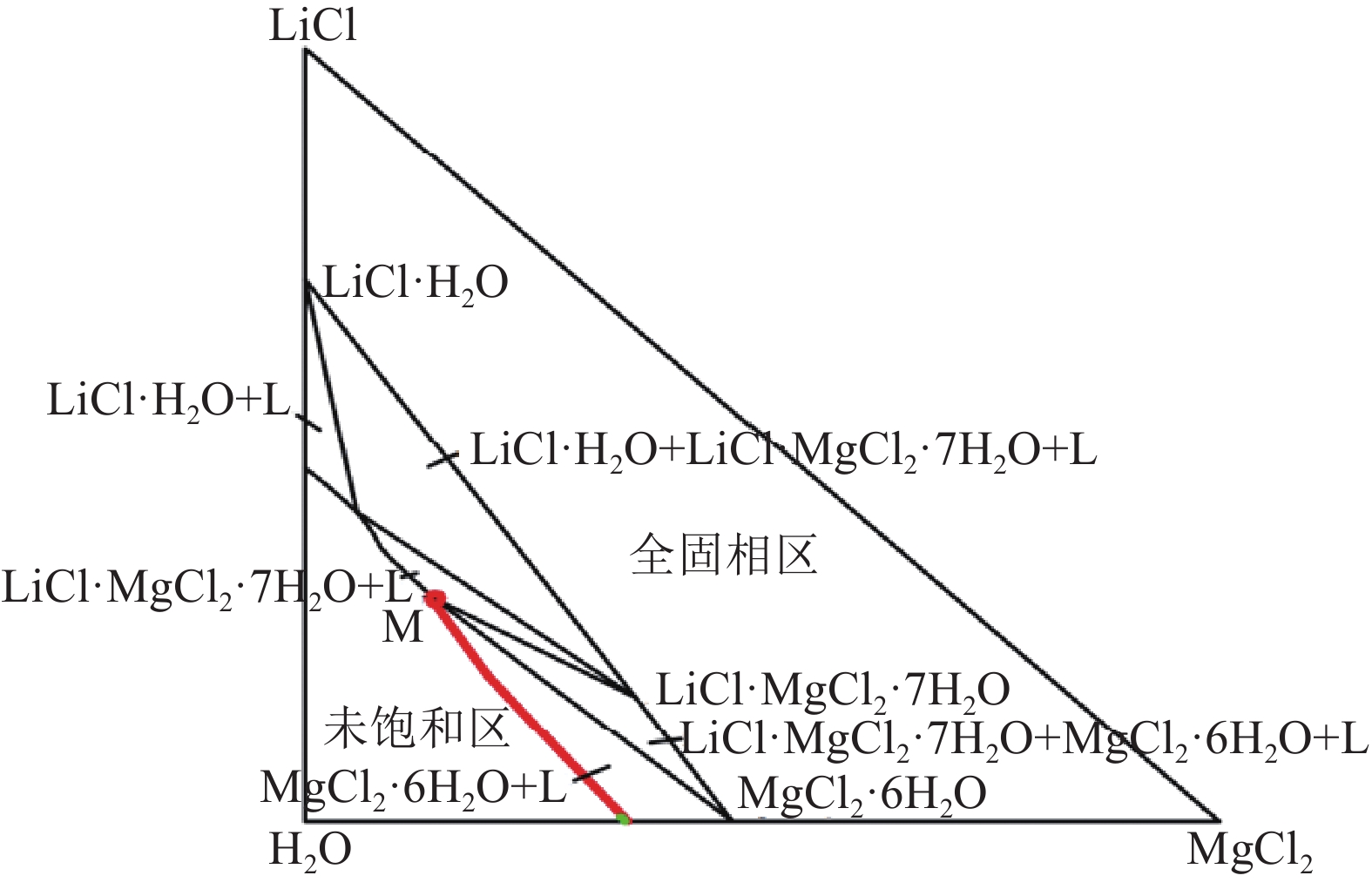
[11] 牛自得, 程芳琴. 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002.NIU Z D, CHENG F Q. Phase diagram of water and salt system and its application[M]. Tianjin: Tianjin University Press, 2022.
NIU Z D, CHENG F Q. Phase diagram of water and salt system and its application[M]. Tianjin: Tianjin University Press, 2022.
[12] 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 第二版. 北京: 科学出版社, 1988.Qinghai Salt Lake Research Institute. Chinese Academy of Sciences. Methods for the analysis of brine and salt[M]. 2nd Ed. Beijing: Science Press, 1988.
Qinghai Salt Lake Research Institute. Chinese Academy of Sciences. Methods for the analysis of brine and salt[M]. 2nd Ed. Beijing: Science Press, 1988.
-



 下载:
下载: