Selective Dissolution and Mechanism of Arsenopyrite by the Oxidizing Agent in Alkaline Leaching System
-
摘要:
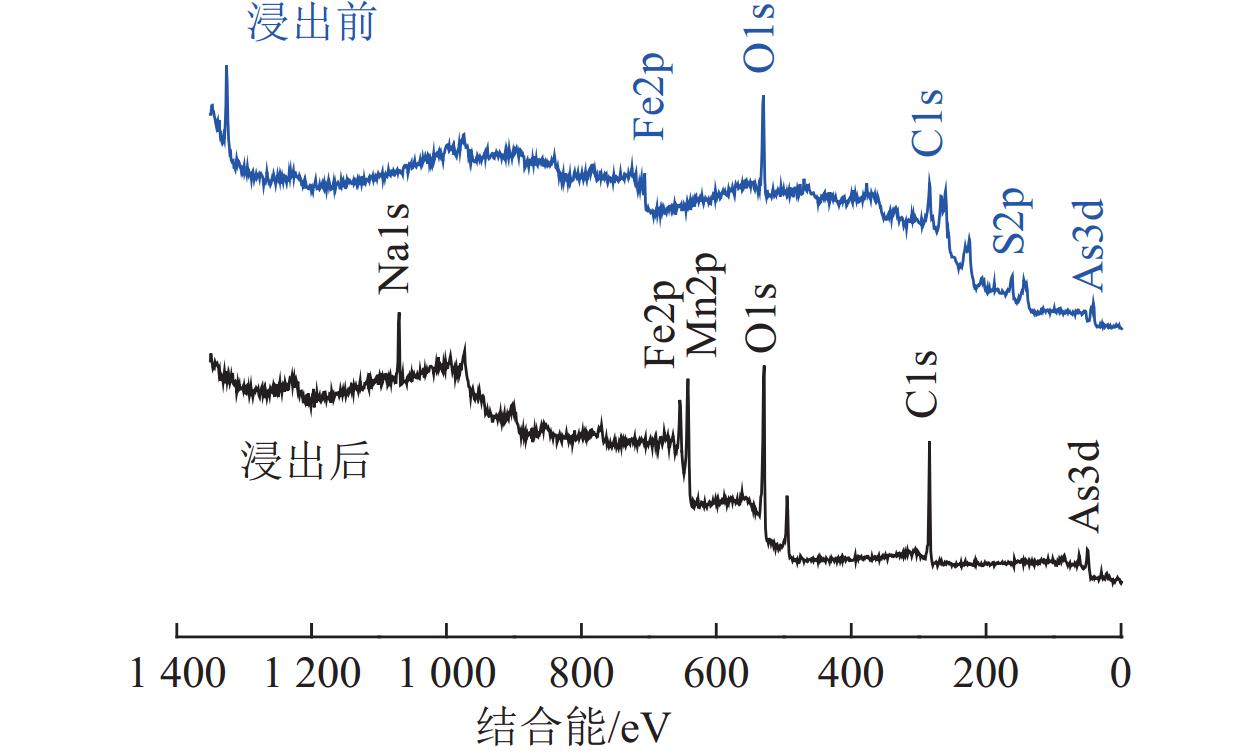
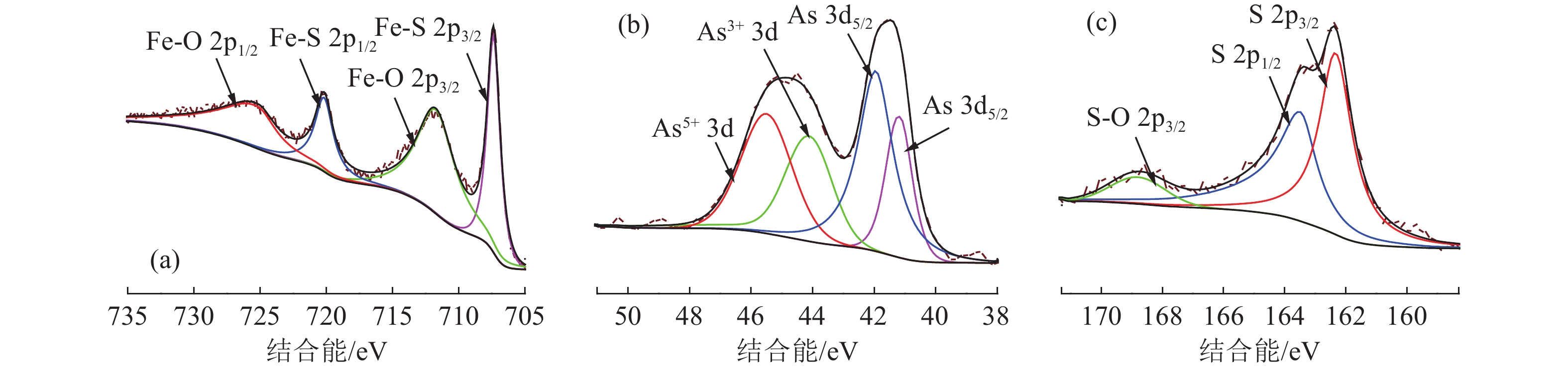
砷黄铁矿是一种常见的载金矿物,砷的存在导致金回收困难。本文研究了砷黄铁矿常压碱浸脱砷过程中,浸出时间、NaOH浓度、温度和高锰酸钾浓度对砷浸出率的影响,并结合电化学、动力学分析,阐明了高锰酸钾的助浸氧化机制。结果表明,当使用0.25 mol/L高锰酸钾作为氧化剂,NaOH浓度为3.5 mol/L时,砷浸出率最高,为63.72%。电化学与动力学分析表明,控制砷黄铁矿常压碱浸反应速率的决定性步骤是化学反应步骤,表观活化能为40.19 kJ/mol,高锰酸钾等氧化剂促进了砷黄铁矿碱浸初期铁的氧化溶解。
Abstract:Arsenopyrite is a common gold-bearing mineral, and the presence of arsenic leads to difficulties in gold recovery. In this paper, the effects of leaching time, NaOH concentration, temperature and potassium permanganate concentration on the arsenic leaching rate in the process of arsenopyrite arsenic removal by atmospheric pressure alkaline leaching were investigated, and the leaching mechanism of potassium permanganate was elucidated by combining electrochemical and kinetic analysis. The results showed that the highest arsenic leaching rate of 63.72% was achieved when 0.25 mol/L potassium permanganate was used as the oxidant and the NaOH concentration was 3.5 mol/L. Electrochemical and kinetic analyses showed that the decisive step controlling the rate of arsenopyrite atmospheric pressure alkali leaching reaction was the chemical reaction step. The apparent activation energy of the reaction is 40.19 kJ/mol. Oxidizing agents such as potassium permanganate promote the oxidative dissolution of iron at the beginning of the alkaline leaching of arsenopyrite.
-
Key words:
- Arsenic removal /
- Arsenopyrite /
- Dissolution /
- Pre-oxidation
-

-
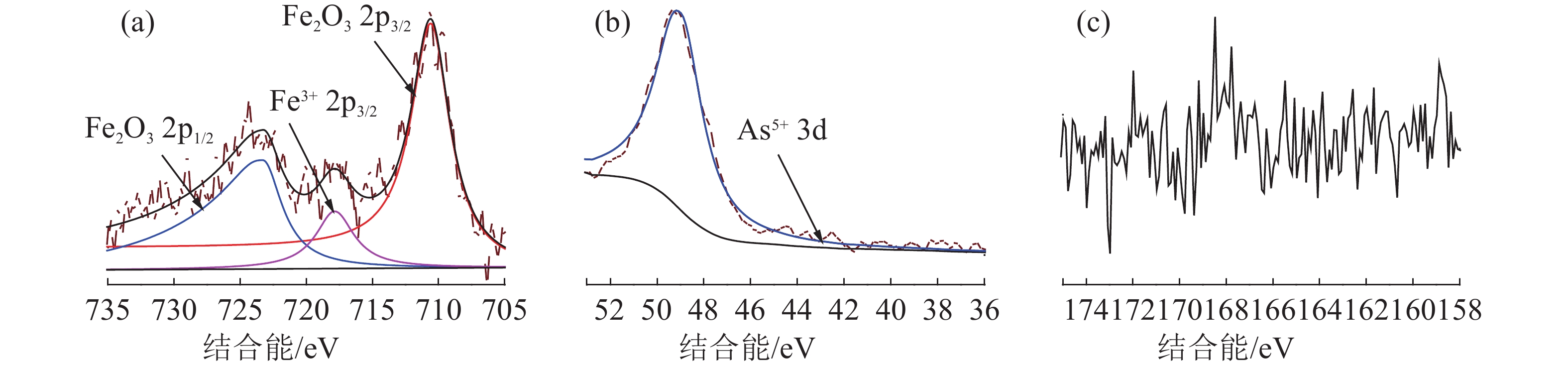
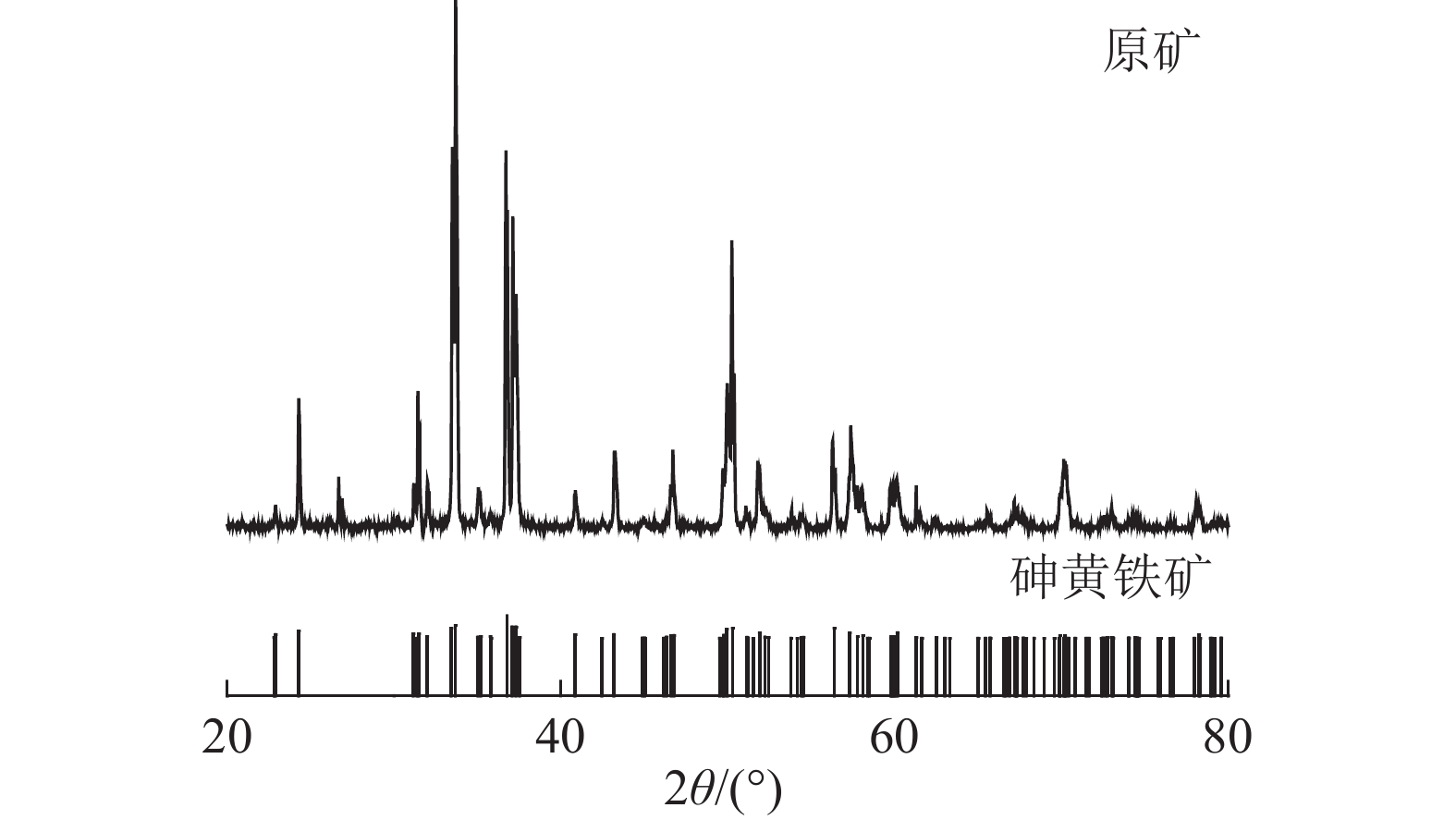
表 1 砷黄铁矿的主要化学成分分析/%
Table 1. Main chemical composition analysis of arsenopyrite
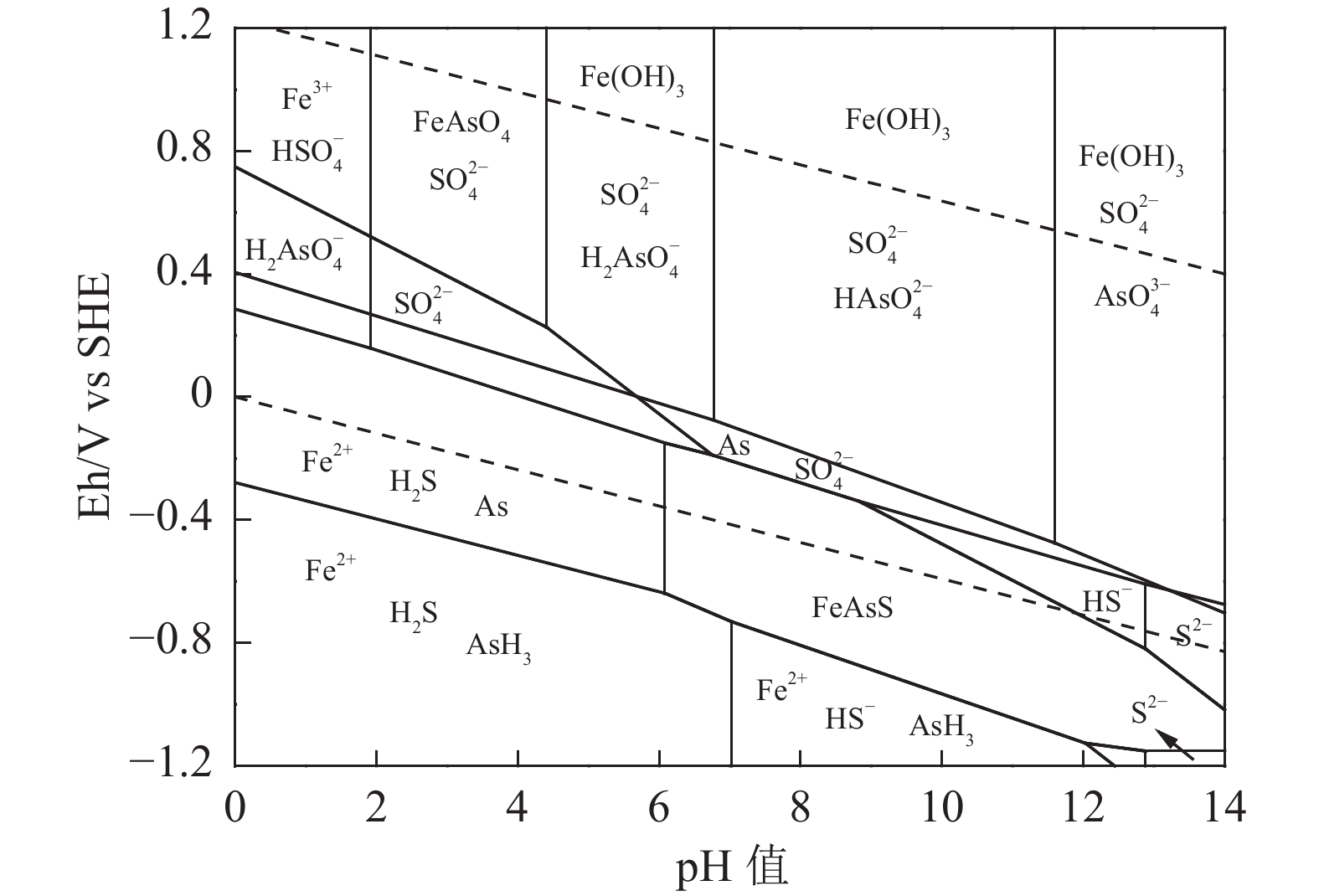
Fe As S 其他 合计 理论 34.30 46.01 19.69 0.00 97.30 实际 33.14 44.78 19.38 2.70 表 2 25 ℃下砷黄铁矿碱浸反应的Eh-pH关系式
Table 2. Eh-pH equations for the alkaline leaching reaction of arsenopyrite at 25 ℃
序号 反应式 Eh-pH关系式 a O2+4H++4e-=2H2O Eh=1.22-0.059pH b H2=2H++2e- Eh=-0.059pH 1 FeAsS+5OH-+3.5O2=Fe(OH)3+AsO43-+SO42-+H2O Eh=1.34-0.021pH 2 14MnO4-+FeAsS+19OH-=14MnO42-+8H2O+AsO43-+Fe(OH)3+SO42- Eh=2.31-0.080pH 3 FeAsS+1.5O2+3H2O=Fe(OH)3+H2AsO3-+S0+H+ Eh=1.18-0.015pH 4 S0+2OH-+1.5O2=SO42-+H2O Eh=1.39-0.020pH 5 H2AsO3-+2OH-+0.5O2=AsO43-+2H2O Eh=1.98-0.059pH 6 4MnO4-+4OH-=4MnO42-+2H2O+O2 Eh=0.98-0.059pH 7 6MnO4-+8OH-+S0=SO42-+4H2O+6MnO42- Eh=2.37-0.079pH 8 2MnO4-+4OH-+H2AsO3-=2MnO42-+3H2O+AsO43- Eh=2.84-0.118pH 表 3 25 ℃时砷黄铁矿碱浸反应的反应平衡常数与吉布斯自由能
Table 3. Equilibrium constants and Gibbs free energy for the alkaline leaching reaction of arsenopyrite at 25 ℃
序号 反应平衡
常数吉布斯自由
能 /(kJ/mol)序号 反应平衡
常数吉布斯自由
能 /(kJ/mol)1 243.664 -1 483.982 5 35.578 -218.387 2 283.445 -1 726.259 6 11.366 -69.222 3 85.933 -523.353 7 125.804 -766.179 4 108.755 -662.347 8 41.261 -251.294 表 4 不同温度和高锰酸钾与氢氧化钠的不同浓度下砷的浸出率/%
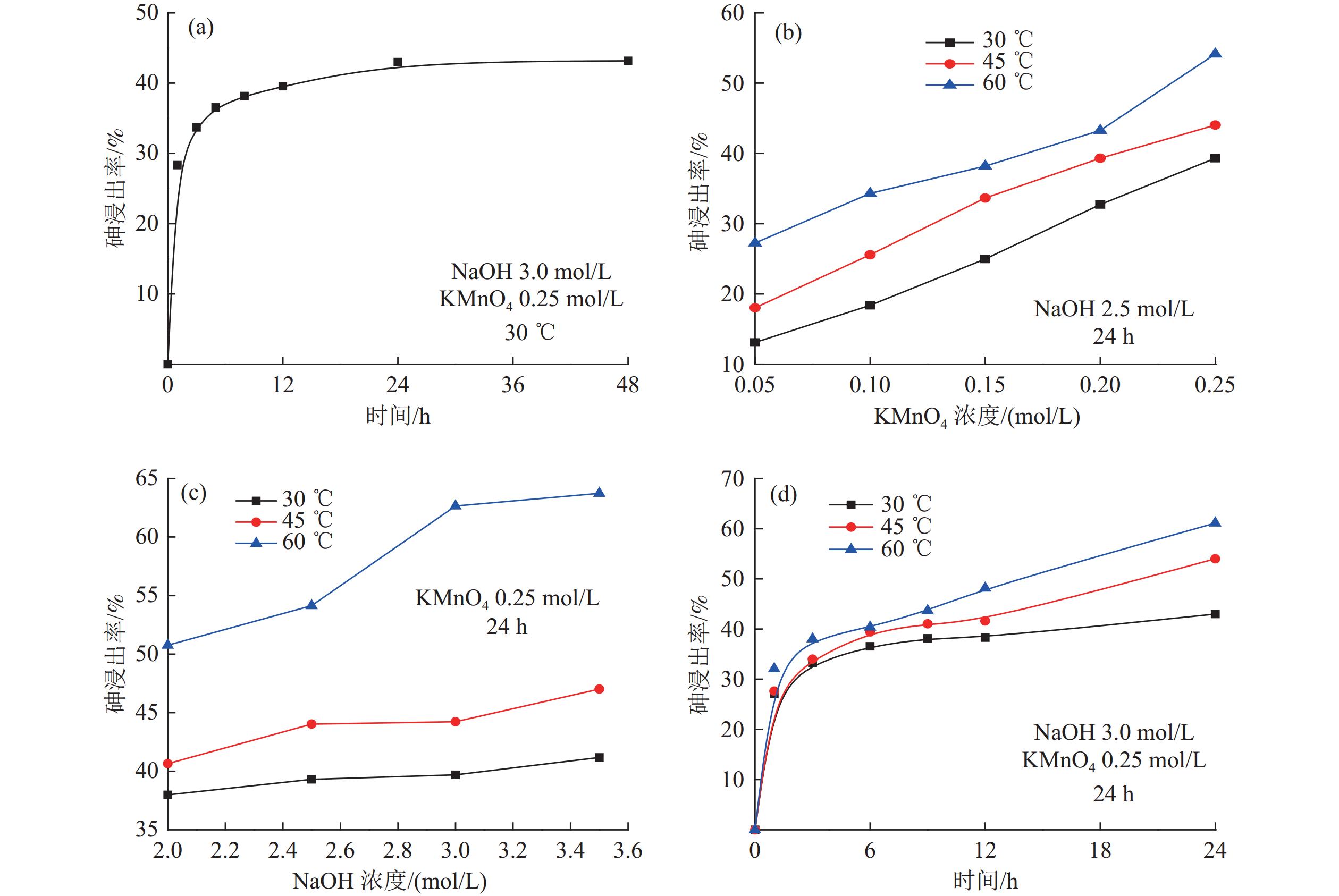
Table 4. Recovery of As at different temperatures and different concentrations of NaOH and KMnO4
药剂种类 用量/(mol/L) 30 ℃ 45 ℃ 60 ℃ KMnO4
(NaOH 2.5 mol/L,24 h)0.05 13.09 18.03 27.25 0.10 18.39 25.57 34.32 0.15 24.97 33.65 38.19 0.20 32.73 39.31 43.27 0.25 39.31 44.03 54.14 NaOH
(KMnO4 0.25 mol/L,24 h)2.00 37.99 40.65 50.76 2.50 39.31 44.03 54.14 3.00 39.70 44.23 62.65 3.50 41.17 47.01 63.72 表 5 不同矿物的拉曼峰峰位
Table 5. Raman band positions of different minerals
矿物种类 化学式 主要拉曼峰
/cm−1次要拉曼峰
/cm−1砷黄铁矿 FeAsS 217,280,392 127,231,333,
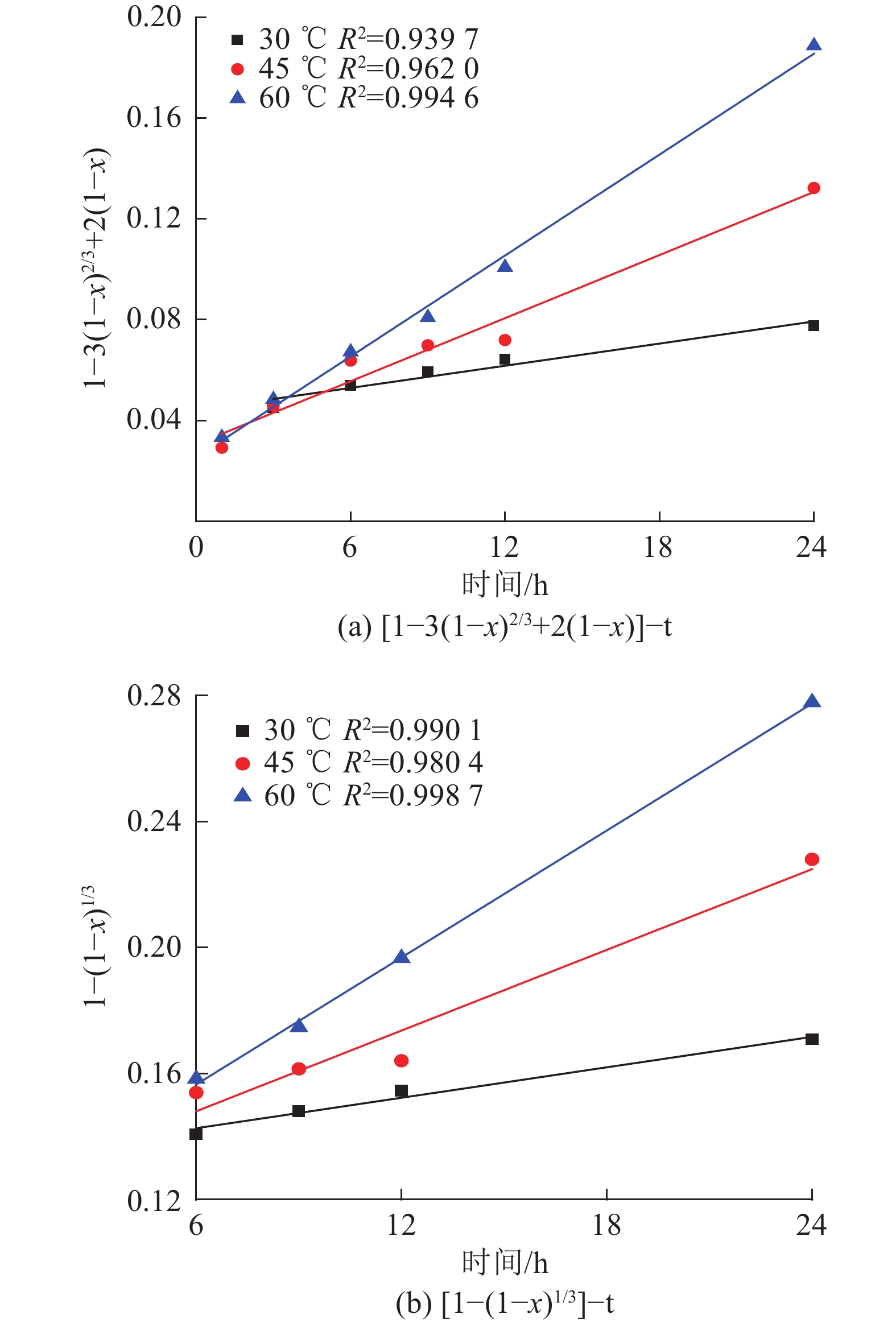
427,453赤铁矿 ɑ-Fe2O3 222,290 230,408,490,607 针铁矿 ɑ-FeO(OH) 297,384 477,545,655 水合氧化铁 5Fe2O3·9H2O 707 361, 508, 1045 雌黄 As2S3 379 152, 289, 200,308 表 6 未反应收缩核模型
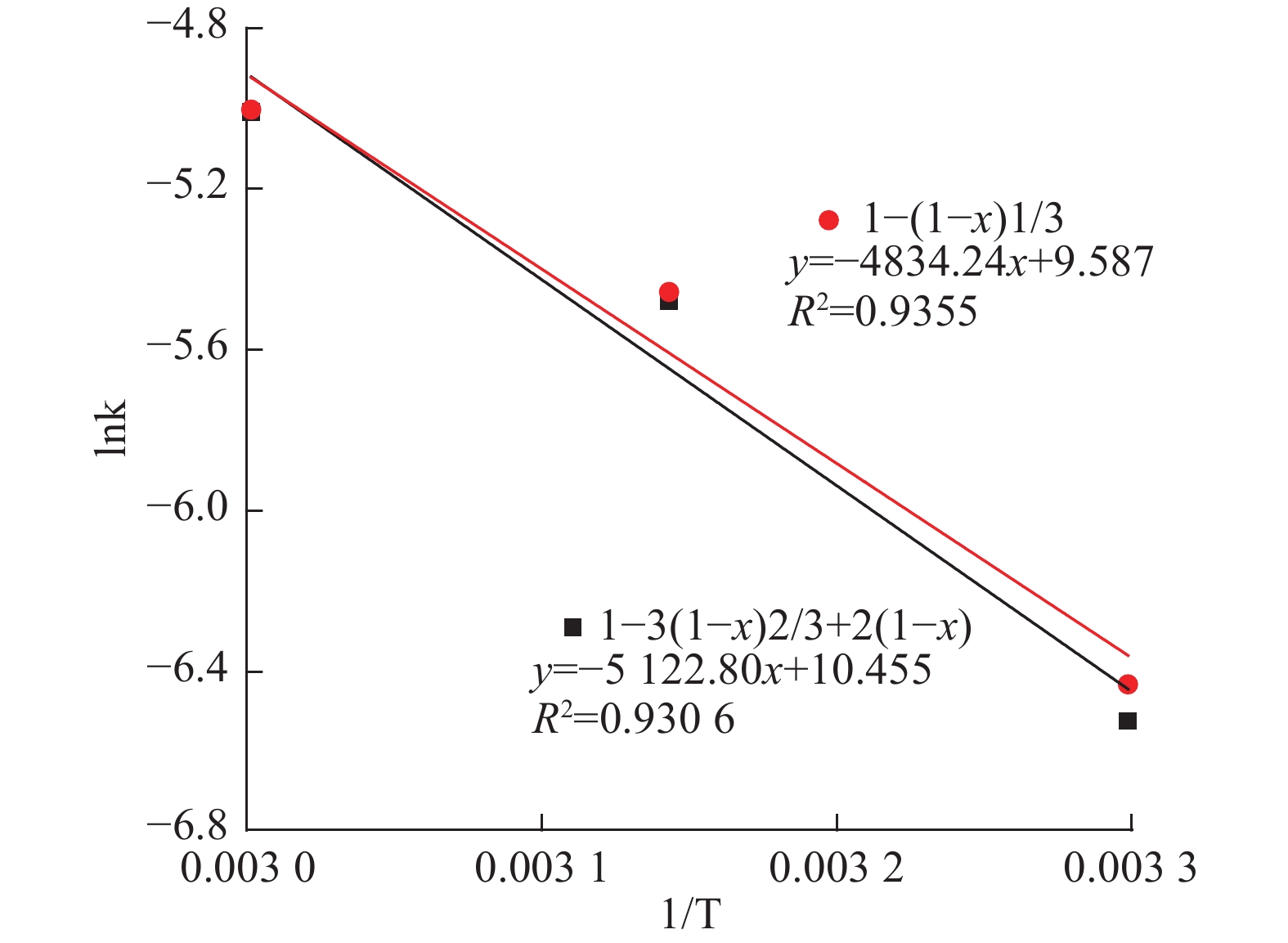
Table 6. Shrinking core model equations
# 控制步骤 方程式 1 液相传质 1-3(1-x)2/3+2(1-x)=kt 2 表面化学反应 1-(1-x)1/3=kt -
[1] 陈京玉, 陈志国, 康卫刚. 新疆某伴生铜钴矿降砷回收工艺研究[J]. 矿产综合利用, 2019(1):51-56.CHEN J Y, CHEN Z G, KANG W G. Research on reducing arsenic and recovering mineral processing technology of certain arsenic-bearing copper ore in Xinjiang[J]. Multipurpose Utilization of Mineral Resources, 2019(1):51-56. doi: 10.3969/j.issn.1000-6532.2019.01.011
CHEN J Y, CHEN Z G, KANG W G. Research on reducing arsenic and recovering mineral processing technology of certain arsenic-bearing copper ore in Xinjiang[J]. Multipurpose Utilization of Mineral Resources, 2019(1):51-56. doi: 10.3969/j.issn.1000-6532.2019.01.011
[2] 胡盘金, 郑永兴, 宁继来, 等. 含砷硫化铜矿浮选除砷研究进展[J]. 矿产综合利用, 2020(5):45-51.HU P J, ZHENG Y X, NING J L, et al. Research progress of arsenic removal from arsenic bearing copper sulphide ore by flotation[J]. Multipurpose Utilization of Mineral Resources, 2020(5):45-51. doi: 10.3969/j.issn.1000-6532.2020.05.005
HU P J, ZHENG Y X, NING J L, et al. Research progress of arsenic removal from arsenic bearing copper sulphide ore by flotation[J]. Multipurpose Utilization of Mineral Resources, 2020(5):45-51. doi: 10.3969/j.issn.1000-6532.2020.05.005
[3] 李磊, 魏旭, 卢晶, 等. 安徽省宣州区茶亭铜多金属矿金赋存状态研究[J]. 矿产综合利用, 2020(2):118-121.LI L, WEI X, LU J, et al. Gold occurrence of chating copper polymetallic deposit of Xuanzhou district, Anhui Province[J]. Multipurpose Utilization of Mineral Resources, 2020(2):118-121. doi: 10.3969/j.issn.1000-6532.2020.02.021
LI L, WEI X, LU J, et al. Gold occurrence of chating copper polymetallic deposit of Xuanzhou district, Anhui Province[J]. Multipurpose Utilization of Mineral Resources, 2020(2):118-121. doi: 10.3969/j.issn.1000-6532.2020.02.021
[4] 李林积, 王丹, 邱鹏玉. 西秦岭格尔托金矿金的赋存状态及可选性试验研究[J]. 矿产综合利用, 2019(4):83-86.LI L J, WANG D, QIU P Y. Experimental study on occurrence and optionality of gold in Gelto gold deposit, western Qinling[J]. Multipurpose Utilization of Mineral Resources, 2019(4):83-86. doi: 10.3969/j.issn.1000-6532.2019.04.017
LI L J, WANG D, QIU P Y. Experimental study on occurrence and optionality of gold in Gelto gold deposit, western Qinling[J]. Multipurpose Utilization of Mineral Resources, 2019(4):83-86. doi: 10.3969/j.issn.1000-6532.2019.04.017
[5] 刘益萍. 提铜降砷的工艺矿物学研究[J]. 矿产综合利用, 2016(1):67-70+75.LIU Y P. Study on the process mineralogy of reducing arsenic in copper[J]. Multipurpose Utilization of Mineral Resources, 2016(1):67-70+75. doi: 10.3969/j.issn.1000-6532.2016.01.016
LIU Y P. Study on the process mineralogy of reducing arsenic in copper[J]. Multipurpose Utilization of Mineral Resources, 2016(1):67-70+75. doi: 10.3969/j.issn.1000-6532.2016.01.016
[6] 李广明, 张洪恩. 硫化矿浮选除砷实践中矿石预处理的影响[J]. 矿产综合利用, 1989(1):45-48.LI G M, ZHANG H E. Effect of ore pretreatment in the practice of arsenic removal from sulphide ore flotation[J]. Multipurpose Utilization of Mineral Resources, 1989(1):45-48.
LI G M, ZHANG H E. Effect of ore pretreatment in the practice of arsenic removal from sulphide ore flotation[J]. Multipurpose Utilization of Mineral Resources, 1989(1):45-48.
[7] 何晓川, 唐晓莲. 毒砂与方铅矿、黄铜矿分离试验研究[J]. 矿产综合利用, 1994(3):16-18.HE X C, TANG X L. Experimental study on the separation of arsenopyrite from galena and chalcopyrite[J]. Multipurpose Utilization of Mineral Resources, 1994(3):16-18.
HE X C, TANG X L. Experimental study on the separation of arsenopyrite from galena and chalcopyrite[J]. Multipurpose Utilization of Mineral Resources, 1994(3):16-18.
[8] MENG Y Q , WU M J, SU S L, et al. Intensified alkiline leaching pretreatment of refractory gold ore at ambient temperature and atmosphere pressure[J]. Nonferrous Metals, 2003(1): 43-47.
[9] 田树国. 高砷金矿常温常压碱浸预处理工艺研究[D]. 赣州: 江西理工大学, 2009.TIAN S G. Study on the pretreatment process of high arsenic gold ore by normal temperature and pressure alkaline leaching[D]. Ganzhou: Jiangxi University of Science and Technology , 2009.
TIAN S G. Study on the pretreatment process of high arsenic gold ore by normal temperature and pressure alkaline leaching[D]. Ganzhou: Jiangxi University of Science and Technology , 2009.
[10] WANG J, WANG W, BAI Y L, et al. Study on pre-oxidation of a high-arsenic and high-sulfur refractory gold concentrate with potassium permanganate and hydrogen peroxide[J]. Transactions of the Indian Institute of Metals, 2020, 73(3):577-586. doi: 10.1007/s12666-020-01863-6
[11] ZHANG Y, Liu RQ, SUN W, et al. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of sodium silicate and sodium sulfite[J]. Transactions of Nonferrous Metals Society of China, 2020, 30(4):1091-1101. doi: 10.1016/S1003-6326(20)65280-3
[12] ASTA M P, PéREZ-LóPEZ R, ROMáN-ROSS G, et al. Analysis of the iron coatings formed during marcasite and arsenopyrite oxidation at neutral-alkaline conditions[J]. Geologica Acta, 2013, 11(4):465-481.
[13] Kharbish S, Andráš P. Investigations of the Fe sulfosalts berthierite, garavellite, arsenopyrite and gudmundite by Raman spectroscopy[J]. Mineralogical Magazine, 2014, 78(5):1287-1300. doi: 10.1180/minmag.2014.078.5.13
[14] Das S, Hendry M J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes[J]. Chemical Geology, 2011, 290(3-4):101-108. doi: 10.1016/j.chemgeo.2011.09.001
[15] Suess E, Planer-Friedrich B. Thioarsenate formation upon dissolution of orpiment and arsenopyrite[J]. Chemosphere, 2012, 89(11):1390-1398. doi: 10.1016/j.chemosphere.2012.05.109
-



 下载:
下载: