Study on the Preparation of Amido Xanthate and Its Flotation Performance for Chalcopyrite and Pyrite
-
摘要:
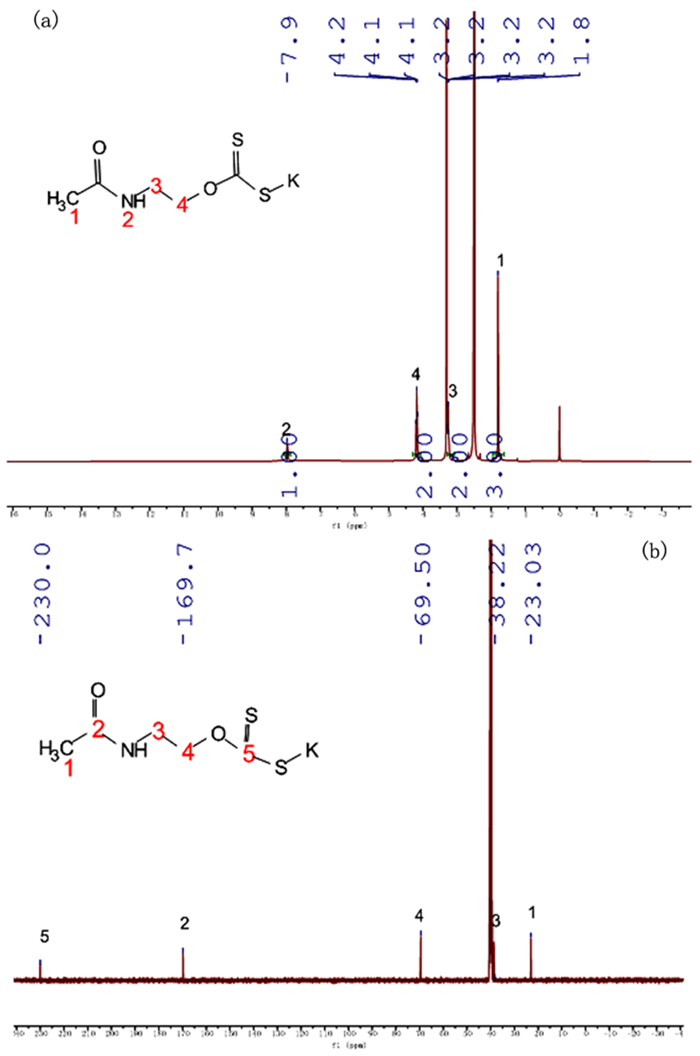
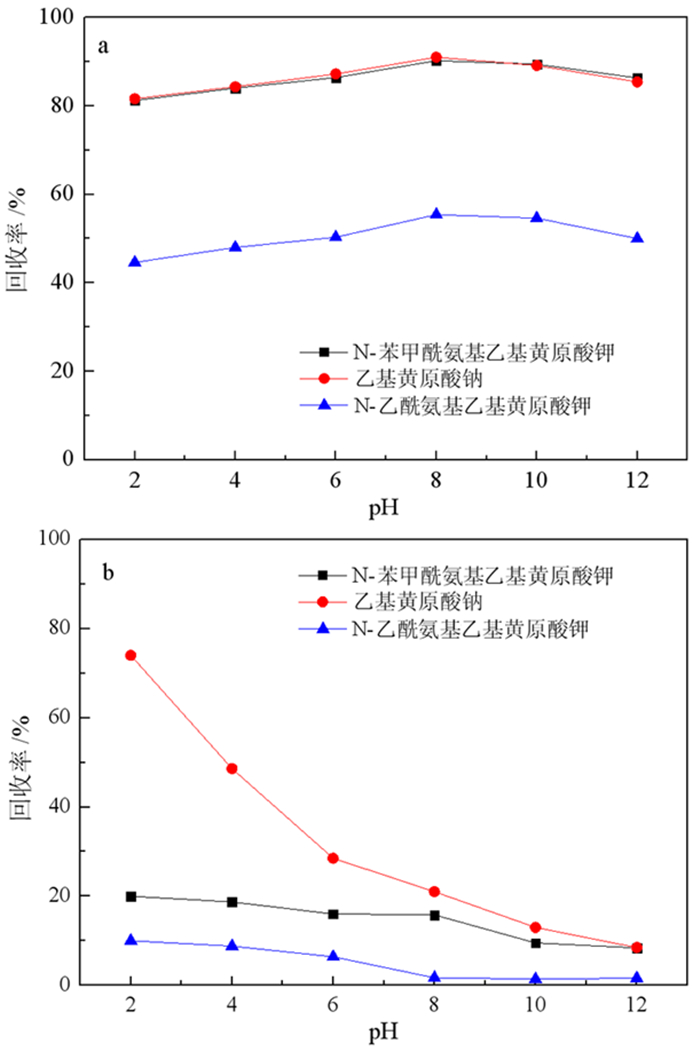
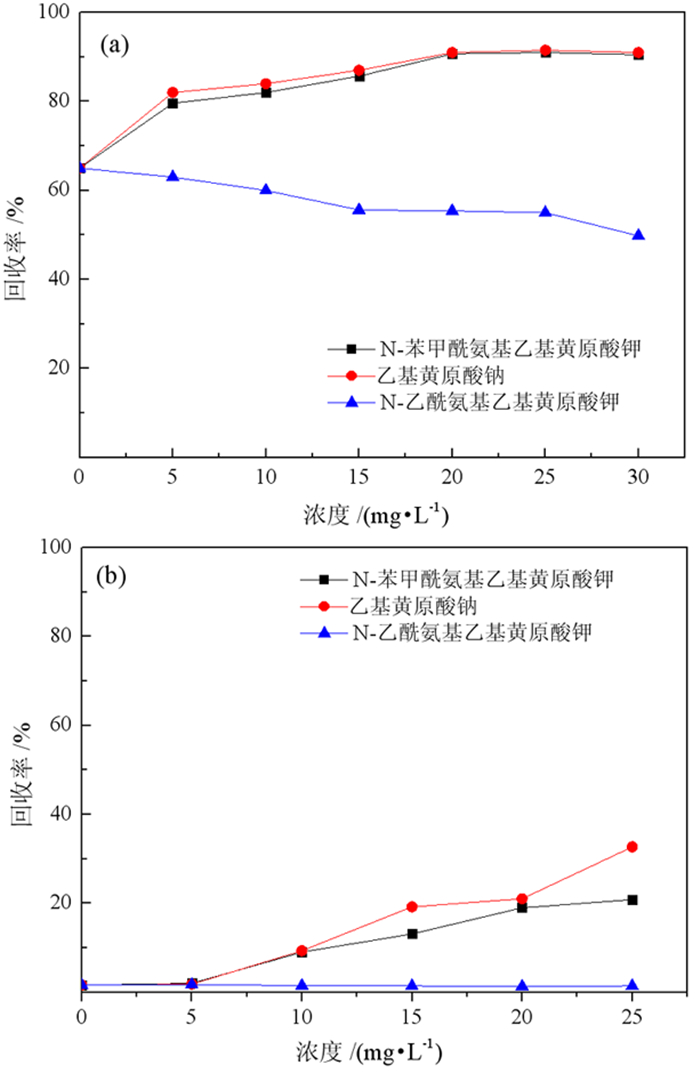
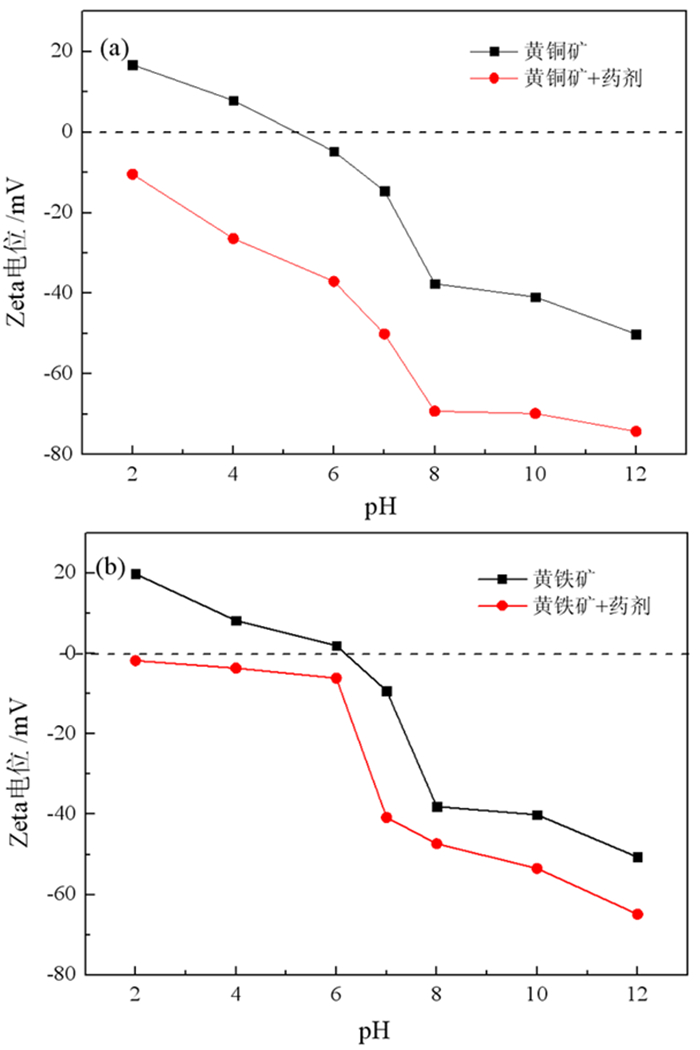
基于浮选药剂分子设计理论和气味分子的结构理论,设计并合成了两种酰氨基黄药—N-乙酰氨基乙基钾黄药和N-苯甲酰氨基乙基钾黄药,并对其结构进行了表征。这种含有酰氨基和黄原酸基的双配体黄药分子间易于产生氢键缔合作用,同时双配体间的相互影响可削弱气味分子在嗅觉感受体表面的定向作用,从而消除分子的恶臭异味,合成试验表明,N-乙酰氨基乙基钾黄药和N-苯甲酰氨基乙基钾黄药均没有刺激性臭味。单矿物浮选试验表明,N-苯甲酰氨基乙基钾黄药对黄铜矿的捕收能力与乙基钠黄药相近,强于N-乙酰氨基乙基钾黄药,对黄铁矿的捕收能力弱于乙基钠黄药,强于N-乙酰氨基乙基钾黄药。吸附量试验表明,捕收剂在黄铜矿表面的吸附量大小顺序与单矿物浮选结果相符合。Zeta电位和红外光谱分析结果表明,酰氨基黄药捕收剂分子在黄铜矿、黄铁矿表面均发生了化学吸附。
Abstract:Based on the molecular design theory of flotation reagents and the structural theory of odor molecules, two amide xanthates—potassium potassium O-(2-acetamidoethyl) carbonodithioate and potassium O-(2-benzamidoethyl) carbonodithioate were designed and synthesized and their structure were characterized.These double ligand xanthates containing amido and xanthate groups were prone to produce hydrogen bond between the molecules, and the interaction between the double ligands could weaken the orienting effect of odor molecules on the surface of the olfactory receptors, thereby eliminating the malodor. The synthesis test showed that both potassium O-(2-acetamidoethyl) carbonodithioate and potassium O-(2-benzamidoethyl) carbonodithioate had no pungent odor of traditional xanthate.The single mineral flotation test demonstrated that the collection performance of potassium O-(2-benzamidoethyl) carbonodithioate was similar to that of ethyl xanthate, but better than that of potassium O-(2-acetamidoethyl) carbonodithioate, and the collection performance of pyrite was weaker than that of ethyl xanthate and stronger than that of potassium O-(2-acetamidoethyl) carbonodithioate.The adsorption capacity test showed that the order of the adsorption amount of the three collectors on the surface of chalcopyrite was consistent with the single mineral flotation results.The results of Zeta potential and infrared spectroscopy further indicated that the amido xanthate collector were chemically adsorbed on the surfaces of chalcopyrite and pyrite.
-
Key words:
- amide xanthate /
- flotation /
- chalcopyrite /
- pyrite /
- DFT calculation
-

-
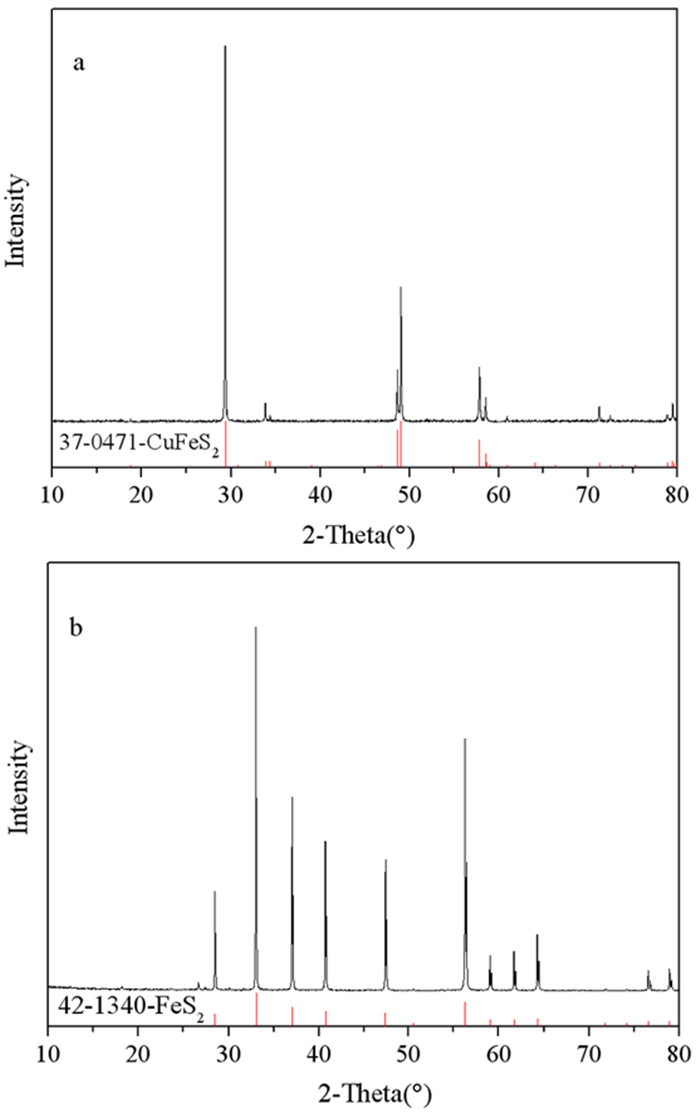
表 1 黄铜矿、黄铁矿中主要元素含量
Table 1. Contents of main elements in chalcopyrite and pyrite
/% Elements Contents/% Chalcopyrite Pyrite Cu 36.02 0.015 Fe 28.62 42.75 S 33.02 49.09 Si 0.409 2.16 Al 0.315 1.14 O 1.5 3.91 表 2 在DFT/B3LYP6-311G+(d)水平下捕收剂的分子前线轨道能量
Table 2. Frontline orbital energy of collectors at DFT/B3LYP6-311G+(d) level
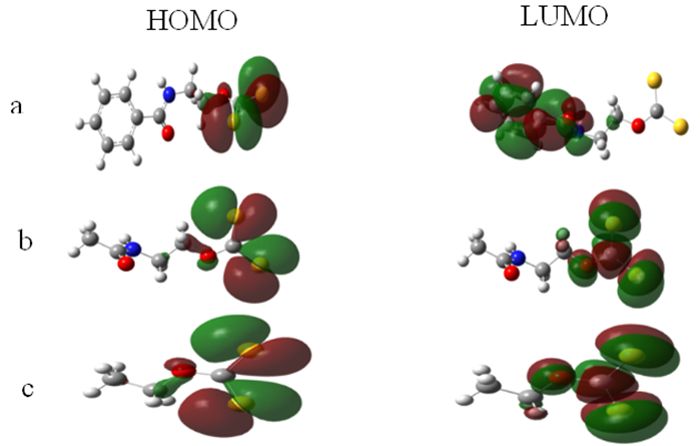
化合物 EHOMO(a.u) ELUMO(a.u) EHOMO-ELUMO(a.u) CLogP N-苯甲酰氨基乙基黄原酸根离子 -0.196 99 -0.051 60 -0.175 69 1.227 N-乙酰氨基乙基黄原酸根离子 -0.196 91 -0.037 65 -0.175 68 -0.478 乙基黄原酸根离子 -0.195 50 -0.035 65 -0.176 47 1.018 表 3 酰氨基黄原酸盐红外光谱吸收峰的归属
Table 3. Assignment of the absorption peaks of acylamino xanthogenate in FTIR spectra
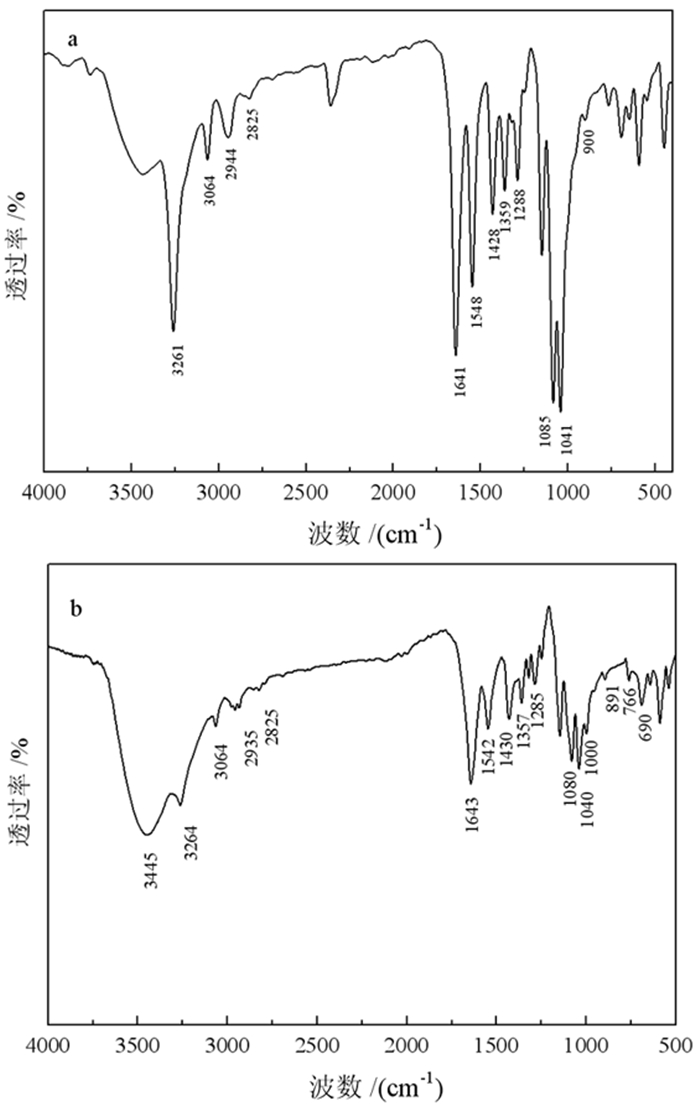
N-乙酰氨基乙基黄原酸钾/cm-1 N-苯甲酰氨基乙基黄原酸钾/cm-1 可能归属 3 261 3 264 N-H的伸缩振动峰 2 944、2 825 2 935、2 825 CH2的伸缩振动峰 1 641 1 643 C=O的伸缩振动峰 1 548 1 542 N-H的变形振动峰 1 288 1 285 C-N的伸缩振动峰 1 085 1 080 C-O-C的伸缩振动峰 1 041 1 040 C=S的伸缩振动峰 900 1 000 C-S的伸缩振动峰 - 891、766、690 苯环上C-H面外弯曲振动峰 -
[1] LIANG S, GUO X, FENG N, et al. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions[J]. Journal of Hazardous Materials, 2009, 170(1): 425-429. doi: 10.1016/j.jhazmat.2009.04.078
[2] 贾云, 钟宏, 王帅, 等. 捕收剂的分子设计与绿色合成[J]. 中国有色金属学报, 2020, 30(2): 456-466. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ202002024.htm
[3] 刘广义, 黄耀国. 黄原酸盐的制备方法: 105384669A[P]. 2016-3-09.
[4] 张俊, 张豫. 粒状黄药的研制与生产技术[J]. 有色矿山, 2000(1): 34-37. doi: 10.3969/j.issn.1672-609X.2000.01.010
[5] 钟宏, 马鑫, 刘广义, 等. 一种粒状黄药的制备方法: 103817014A[P]. 2014-05-27.
[6] M.G.J. BEETS. Structure and Odour: In Molecular Structure and Organoleptic Quality, Society of Chemical Industry[J]. Structure-Response Relationships in Chemoreception, 1957, 124: 54-90. http://agris.fao.org/openagris/search.do?recordID=US201300343682
[7] MAKHLOUF M M, RADWAN A S, GHAZAL B. Experimental and DFT insights into molecular structure and optical properties of new chalcones as promising photosensitizers towards solar cell applications[J]. Applied Surface Science, 2018, 452: 337-351. doi: 10.1016/j.apsusc.2018.05.007
[8] YANG X, HUANG Y, LIU G, et al. A DFT prediction on the chemical reactivity of novel azolethione derivatives as chelating agents: Implications for copper minerals flotation and copper corrosion inhibition[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 109-123. doi: 10.1016/j.jtice.2018.09.022
[9] ZHAO G, ZHONG H, QIU X, et al. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation[J]. Minerals Engineering, 2013, 49: 54-60. doi: 10.1016/j.mineng.2013.04.025
[10] WALTERS M A, BARAD J, SIRECI A, et al. Xanthate sulfur as a hydrogen bond acceptor: the free xanthate anion and ligand sulfur in nickel tris ethylxanthate[J]. Inorganica Chimica Acta, 2005, 358(3): 633-640. doi: 10.1016/j.ica.2004.09.036
[11] DENG L, ZHAO G, ZHONG H, et al. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation[J]. Journal of Industrial and Engineering Chemistry, 2016, 33: 131-141. doi: 10.1016/j.jiec.2015.09.027
[12] JIA Y, HUANG X, HUANG K, et al. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces[J]. Journal of Industrial and Engineering Chemistry, 2019, 77: 416-425. doi: 10.1016/j.jiec.2019.05.005
[13] LIU S, LIU G, ZHONG H, et al. The role of HABTC's hydroxamate and dithiocarbamate groups in chalcopyrite flotation[J]. Journal of industrial and engineering chemistry, 2017, 52: 359-368. doi: 10.1016/j.jiec.2017.04.015
[14] ZHANG Y, CAO Z, CAO Y, et al. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces[J]. Journal of Molecular Structure, 2013, 1048: 434-440. doi: 10.1016/j.molstruc.2013.06.015
[15] 马鑫, 王帅, 钟宏. 苄基三硫代碳酸钠的合成及其对黄铜矿的浮选性能[J]. 中国有色金属学报, 2018, 28(5): 1067-1075. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201805024.htm
[16] XIAO J, DI N, LIU G, et al. The interaction of N-butoxypropyl-N'-ethoxycarbonylthiourea with sulfide minerals: Scanning electrochemical microscopy, diffuse reflectance infrared Fourier transform spectroscopy, and thermodynamics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 456: 203-210. http://www.sciencedirect.com/science/article/pii/S0927775714004890
[17] DUARTE-HERNáNDEZ A M, CONTRERAS R, SUáREZ-MORENO G V, et al. New 4-hydroxy-N-(2-hydroxyethyl) butanamides: Structure and acidity[J]. Journal of Molecular Structure, 2015, 1081: 146-158. doi: 10.1016/j.molstruc.2014.10.012
[18] FRANZ M, STALLING T, SCHAPER R, et al. Facile Access to Amido (Thio) xanthates under Eco-Friendly Conditions by One-Pot Three-Component Reaction (3-CR)[J]. Synthesis, 2017, 49(17): 4045-4054. doi: 10.1055/s-0036-1589050
[19] JIA Y, HUANG X, HUANG K, et al. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces[J]. Journal of Industrial and Engineering Chemistry, 2019, 77: 416-425. doi: 10.1016/j.jiec.2019.05.005
[20] HUANG X, JIA Y, WANG S, et al. Novel sodium O-benzythioethyl xanthate surfactant: synthesis, DFT calculation and adsorption mechanism on chalcopyrite surface[J]. Langmuir, 2019, 35(47): 15106-15113. doi: 10.1021/acs.langmuir.9b03118
[21] DENG L, WANG S, ZHONG H, et al. A novel surfactant 2-amino-6-decanamidohexanoic acid: Flotation performance and adsorption mechanism to diaspore[J]. Minerals Engineering, 2016, 93: 16-23. doi: 10.1016/j.mineng.2016.04.002
[22] 于宏东, 孙传尧. 不同成因类型黄铁矿的浮游特性[J]. 有色金属, 2009, 61(3): 111-115. doi: 10.3969/j.issn.2095-1744.2009.03.028
[23] MITCHELL T K, NGUYEN A V, EVANS G M. Heterocoagulation of chalcopyrite and pyrite minerals in flotation separation[J]. Advances in colloid and interface science, 2005, 114: 227-237. http://europepmc.org/abstract/MED/15894282
[24] GAUDIN A M, SUN S C. Correlation between mineral behavior in cataphoresis and in flotation[J]. Metallurgical and Materials Transactions, A. Physical Metallurgy and Materials Science, 1946, 169: 347-362.
-



 下载:
下载: