Determination of Chloride and Sulfate in Trona Ores by Ion Chromatography
-
摘要: 通过测定氯离子和硫酸根离子可以确定天然碱矿中石盐和芒硝的含量,目前分析方法比较少,且流程冗长,容易污染,生产效率低。本文建立了离子色谱-抑制电导法测定天然碱矿中氯离子和硫酸根的分析方法。样品中水溶性阴离子经热水溶解进入溶液,以30 mmol/L氢氧化钾溶液作为淋洗液,利用AG19阴离子保护柱、AS19阴离子分离柱分离样品中的氯和硫酸根,CRD 200碳酸盐消除装置去除了碳酸根的干扰。方法检出限氯离子为0.01 mg/L,硫酸根为0.02 mg/L;加标回收率为100.9%~104.1%;精密度(RSD,n=10)小于2.5%。实际样品分析结果与滴定法的测定值基本吻合。本法操作简单,重现性好,灵敏度高,结果可靠,不受样品复杂组分及形成缓冲溶液体系的干扰,很好地解决了传统方法费时、耗材、不能同时测定氯离子和硫酸根的问题。Abstract: Through the determination of chloride and sulfate, the contents of salt and sodium sulfate in natural trona ore can be obtained. At present analysis methods are inefficient, causing disadvantages, such as having a time-consuming process, easy pollution and low production efficiency. This study establishes the analysis method which detects chloride and sulfate in natural trona ore by the ion chromatography-suppression conductivity measurement. The anions were dissolved into the solution by hot water and the chloride and sulfate were separated by using AG-19 type anion protect column and AS-19 type anion separation column with 30 mmol/L KOH as the eluent. The interference from carbonate ion was eliminated by a Carbonate Removal Device (CRD). Under optimal conditions, the detection limits of chloride and sulfate were 0.01 and 0.02 mg/L, respectively. The recoveries were between 100.9%-104.1% and the precisions of the method (RSD, n=10) were less than 2.5%. The results for the actual samples were consistent with those obtained by the titration method. This method is simple and avoids interferences by the complicated components of the sample and the buffer solution system, while also being economical, accurate, giving good reproducibility and high sensitivity.
-
Key words:
- trona ores /
- chloride /
- sulfate /
- Ion Chromatography
-

-
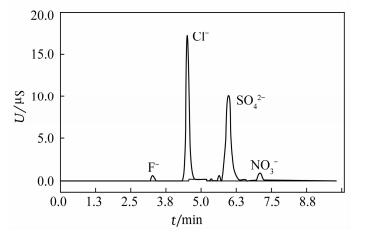
表 1 不同淋洗液离子的保留时间及分离度
Table 1. Retention time and separating degree of different eluent ions
淋洗液离子 指标 氢氧化钾淋洗液浓度
c(KOH)/(mmol·L-1)20 25 30 35 Cl- tR/min 4.68 4.28 4.36 4.12 RS 2.87 3.04 3.57 3.30 SO42- tR/min 6.26 5.99 5.91 5.42 RS 1.98 4.79 6.41 6.45 表 2 方法检出限和测定范围
Table 2. Detection limit and determinaiton range of the method
阴离子 测定范围
ρ/(mg·L-1)线性方程 相关系数 检出限/
(mg·L-1)Cl- 0.1~200 y=0.2938x-0.1837 0.9999 0.01 SO42- 0.2~200 y=0.2151x-0.0177 1.0000 0.02 表 3 方法精密度
Table 3. Precision tests of the method
样品测定
次数Cl- SO42- A/(μS·min) ρ/(mg·L-1) A/(μS·min) ρ/(mg·L-1) 1 8.51 30.05 4.679 22.8 2 8.62 30.42 4.815 23.43 3 8.803 31.03 4.759 23.17 4 8.519 30.08 4.784 23.28 5 8.73 30.79 4.524 22.08 6 8.587 30.31 4.633 22.58 7 8.883 31.3 4.813 23.42 8 8.718 30.75 4.715 22.96 9 8.636 30.47 4.598 22.42 10 8.791 30.99 4.691 22.85 平均值 8.68 30.62 4.701 22.9 RSD/% 1.37 1.30 2.04 1.84 表 4 加标回收试验
Table 4. Recovery tests of the method
元素 ρ/(mg·L-1) 回收率/% 平均
回收率/%样品含量 加标量 回收量 Cl- 7.69 8.00 8.07 100.9 101.9 16.00 16.39 102.4 40.00 41.00 102.5 SO42- 6.08 8.00 8.33 104.1 103.3 16.00 16.44 102.8 40.00 41.21 103.0 表 5 分析结果比对
Table 5. Comparison of analytical results of elements in samples
样品编号 w(Cl-)/% w(SO42-)/% 本法 硝酸银滴定法 本法 EDTA滴定法 样品1 1.57 1.63 3.75 3.71 样品2 2.38 2.44 8.14 8.37 样品3 10.65 10.58 5.67 5.58 样品4 4.43 4.42 2.35 2.24 样品5 5.86 5.70 1.87 1.92 样品6 0.36 0.45 12.65 12.78 -
[1] 河南省地质局实验室.矿物原料分析[M].1974.
[2] 张晓梅.天然碱矿中硫酸根含量的快速测定[J].化工矿物与加工,2004(8): 32-34. http://www.cnki.com.cn/Article/CJFDTOTAL-HGKJ200408013.htm
[3] 王萍.电位滴定法测定天然碱中的Cl-[J].纯碱工业,1988(1): 41-43.
[4] 段胜利.天然碱仪器分析[J].纯碱工业,1984(1): 65. http://www.cnki.com.cn/Article/CJFDTOTAL-CJGY198401025.htm
[5] 王梅英.天然碱中碳酸钠、碳酸氢钠、总碱度的分析方法研究[J].南阳师范学院学报:自然科学版,2004,3(9): 47-50. http://www.cnki.com.cn/Article/CJFDTOTAL-NYSF200409014.htm
[6] 吴伟杰,王恒.测定味精中硫酸盐的离子色谱法[J].浙江预防医学,2012,24(3): 93-94. http://www.cnki.com.cn/Article/CJFDTOTAL-DZJI200911046.htm
[7] 孙明山,孙忠萍,牛朝红,王艳玲,赵保成,李琳.离子色谱法对生活饮用水中氟、氯、硫酸根、硝酸根阴离子的测定[J].农业与技术, 2004,27(4): 89-90. http://www.cnki.com.cn/Article/CJFDTOTAL-NYYS200702030.htm
[8] 蔡茜.离子膜电渗析法在天然碱卤水脱盐中的应用及离子色谱分析研究[D].北京:北京化工大学, 2012.
[9] 郭开强.离子色谱测定岩盐样品中硫酸根及氯离子方法[J].新疆有色金属, 2011(1): 46-47. http://www.cnki.com.cn/Article/CJFDTOTAL-XJYS201101015.htm
[10] 王敬花,张锦梅,胡加鹏.氧弹燃烧-离子色谱法测定海藻酸钠中氯离子和硫酸根离子[J].化学分析计量,2011,20(6): 30-32. http://www.cnki.com.cn/Article/CJFDTOTAL-HXFJ201106012.htm
[11] 叶明立,朱岩,施青红.离子色谱法测定有机溶剂中痕量阴离子[J].分析化学,2005,33(2): 187-190. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX200502009.htm
[12] 杨海军,丁明玉.抑制型离子色谱测定纳米金刚石粉末表面吸附的阴离子[J].分析化学,2002,30(12): 1497-1500. doi: 10.3321/j.issn:0253-3820.2002.12.023
[13] 陈梅兰,焦霞,朱岩.离子色谱法测定环丁砜中痕量阴离子[J].理化检验:化学分册,2008,44(5): 409-413. http://www.cnki.com.cn/Article/CJFDTOTAL-LHJH200805009.htm
[14] 徐咏薇,王海波.离子色谱法测定高浓度碳酸盐基质中痕量阴离子[J].中国卫生检验杂志,2008(6): 1037-1038. http://www.cnki.com.cn/Article/CJFDTOTAL-ZWJZ200806026.htm
[15] 周丽淅,王芳,苏瑾.热水解-离子色谱法测定连续测定地质物料中的氟、氯和硫[J].物探与化探,1989,13(1): 61-65. http://www.cnki.com.cn/Article/CJFDTOTAL-WTYH198901010.htm
[16] 王红伟,刘俊娓,路凯,林少彬.离子色谱法同时测定水中的五种阴离子的研究[J].现代科学仪器,2007(2): 103-105. http://www.cnki.com.cn/Article/CJFDTOTAL-XDYQ200702029.htm
[17] 周爽,于泓,艾红晶.直接电导检测离子色谱法分离测定氟硼酸根及常见无机阴离子[J].分析化学,2008,36(11): 1521-1525. doi: 10.3321/j.issn:0253-3820.2008.11.013
-



 下载:
下载: