Application of a New Silica Gel Ionization Intensifier on Nanogram Lead Isotope Analysis
-
摘要: 核取证分析中需要实施纳克级(ng)铅的同位素全谱分析,质谱测量中要求铅的电离效率较高(大于10-3)。传统硅胶-磷酸技术多用于微克级(μg)铅分析,电离效率一般为10-4~10-3;尽管后来发展的硅胶-硼酸技术可以得到10-3以上的发射效率,但是由于铅在电离过程中存在离子流反复升高-衰减的过程,导致离子流发射不稳定,质谱测量条件难以掌握。本文改进了传统的硅胶发射剂,建立了一种新型硅胶加载体系——硅胶-高铼酸体系。与硅胶-硼酸技术相比,采用硅胶-高铼酸体系可显著提高铅的发射效率并获得稳定的离子流。对于1 ng铅的同位素质谱全谱测量,204Pb/206Pb、207Pb/206Pb、208Pb/206Pb的相对标准偏差分别为0.4%、0.2%和0.1%,测试精度优于采用其他硅胶技术。通过实验比较了硅胶-高铼酸体系与多种传统硅胶发射剂体系对铅离子流的发射效果,优化了硅胶试剂用量为0.5~3.0 μL,采用“夹心饼干”的涂样顺序,对1~100 ng铅样品的发射效率达到6.0×10-3~4.6×10-2,比传统硅胶-磷酸体系的发射效率(10-4~10-3)高了近10倍,与硅胶-硼酸技术的发射效率相当(10-3~10-2),但铅离子发射更为稳定。本文建立的硅胶-高铼酸体系在测量精度上可满足核取证研究中铅作为地域指示剂的需求。Abstract: In nuclear forensics the amount of Pb to be analyzed is usually in the order of several nanograms; that is >10-3 of ionization efficiency is required for mass spectrometry measurements. The traditional silica-gel-phosphoric acid loading technique is typically used to measure microgram amounts of Pb, and the ionization efficiency is in the order of 10-4-10-3. Although the boron-silica gel technique could increase the ionization efficiency to 10-3 or higher, it is also difficult to handle measurement conditions as the ion flow repeatedly increased and decay process in the ionization of Pb resulting in the ion emission instability. A new silica gel-perrhenic acid loading reagent method has been developed and exhibits a significant enhancement in the ionization efficiency of Pb. Compared with the boron-silica gel technique, the emission of Pb ions is very stable with this new technique. The measurement Relative Standard Deviation of 204Pb/206Pb, 207Pb/206Pb, 208Pb/206Pb was 0.4%, 0.2% and 0.1%, respectively from 1 ng of pb, which is an improvement over the precision with other silica gel techniques. The enhancement effect on the ion emission of Pb was compared with the silica gel-perrhenic acid and some other traditional silica gel techniques. The reagent amount of the silica gel was optimized to 0.5-3.0 μL and the ‘sandwich biscuit’ loading sequence was used. The ionization efficiency of the Pb reached 6.0×10-3-4.6×10-2 for 1100 ng Pb. The ionization efficiency was close to the boron-silica gel technique, whereas it was about ten times higher than the traditional silica gel-phosphoric acid technique. A stable ion current was obtained by use of the silica gel-perrhenic acid technique, and the measurement accuracy meets the requirements for region indicators on nuclear forensic analysis.
-

-
表 1 硅胶用量选择
Table 1. Ion current of 208Pb with different dosage of silica gel
硅胶用量
(μL)208Pb离子流
(105 cps)测量时间
(min)0.5 5.5~31 35 1.0 4.6~45 42 2.0 2.5~51 50 3.0 3.8~30 52 4.0 1.2~4.3 72 5.0 0.6~1.1 90 表 2 硅胶-高铼酸体系下铅的质谱测量效率
Table 2. The total ionization efficiency of 208Pb with silica gel-HReO4 system
样品 样品用量
(ng)测量时间
(h)208Pb离子流
平均强度(cps)样品中208Pb
理论原子数质谱测量
电离效率SRM 981
铅同位素
标准物质1 10.1 6.8×105 1.45×1012 4.6×10-2 2 12.0 6.9×105 2.9×1012 1.0×10-2 25 18.4 3.8×106 3.7×1013 6.0×10-3 100 16.3 5.0×107 1.5×1014 1.8×10-2 204Pb
稀释剂
标准物质10 11.1 9.9×105 3.5×1012 1.0×10-2 20 12.5 2.0×106 7.0×1012 9.9×10-3 50 17.4 2.5×107 1.7×1012 9.0×10-3 表 3 SRM 981铅同位素标准物质测量结果
Table 3. The measurement results of Pb isotope standard material SRM 981
样品量
/ng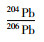
RSD
(%)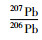
RSD
(%)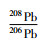
RSD
(%)1 0.05900 0.4 0.9121 0.2 2.171 0.1 10 0.05914 0.2 0.9100 0.1 2.159 0.1 25 0.05917 0.1 0.9096 0.1 2.161 0.0 标称值 0.05962 0.3 0.9082 0.1 2.147 0.4 -
[1] Svedkauskaite-LeGore J, Mayer K, Miller D L, Riciputi L R, Horita J, Bostock D A. Analysis of Concentrated Uranium Ore Using Stable Isotopes and Elemental Concentrations[C]//Fall Meeting of American Geophysical Union. San Francisco. 2006:11-15.
[2] Svedkauskaite-LeGore J.Development and validation of a method for origin determination of uranium-bearing material[D].Vilnius: University of Vilnius, 2007:34-44.
[3] Smith D K. Identifying the source of stolen nuclear materials[J].Science and Technology Review, 2007,1:12-18.
[4] Varga Z. Application of lead and strontium isotope ratio measurements for the origin assessment of uranium ore concentrates[J]. Analytical Chemistry, 2009, 81(20): 8327-8334. doi: 10.1021/ac901100e
[5] Bollhoer A. Stable lead isotope ratios and metals in freshwater mussels from a uranium mining environment in Australia's wet-dry tropics[J]. Applied Geochemistry, 2012,27:171-185. doi: 10.1016/j.apgeochem.2011.10.002
[6] Frostick A, Bollh fer A, Parry D, Munksgaard N, Evans K. Radioactive and radiogenic isotopes in sediments from Cooper Creek, Western Arnhem Land[J]. Journal of Environmental Radioactivity, 2008,99(3): 468-482. doi: 10.1016/j.jenvrad.2007.08.015
[7] 杨红梅,路远发,吕红,刘焰,章丽娟,鲍征宇.土壤及其他背景样品中铅同位素比值的测定方法[J].分析化学,2005, 33(11):1603-1606. doi: 10.3321/j.issn:0253-3820.2005.11.023
[8] 王琬,刘咸德,鲁毅强,郭冬发.北京冬季大气颗粒物中铅的同位素丰度比的测定和来源研究[J].质谱学报,2002,23(1):21-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZPXB200201004.htm
[9] 罗若荣,刘学宁,李晴.食品与环境中铅对儿童血铅水平的影响分析[J].广东微量元素科学,2009(5):38-42. http://www.cnki.com.cn/Article/CJFDTOTAL-GWYS200205007.htm
[10] 孟宪厚.提高质谱分析灵敏度的若干方法[J].分析仪器,1994(1):1-5. http://www.cnki.com.cn/Article/CJFDTOTAL-FXYQ199401000.htm
[11] 董灵英,孟宪厚.核纯铀和铀化合物中微量和超微量杂质元素分析的新技术[M].北京:原子能出版社,1998:271.
[12] 刘琦. Pb的高精密度质谱分析[C]//第五次全国同位素质谱年会.北京:中国质谱学会,1985:23-25.
[13] 黄达峰,邓中国.同位素质谱分析技术[M].北京:化学工业出版社,2006:73-74.
[14] 孟宪厚,黄达峰.同位素稀释质谱法测定U3O8中痕量铅[J].铀矿冶,1988,3(7):43-48.
[15] 闫秋实.成都市区河道淤泥的铅同位素组成特征及铅污染来源研究[D].成都:成都理工大学,2004.
[16] 高博,涂湘林,刘颖,孙可,湖光黔,曾文. AG-MP-1M阴离子交换树脂分离-表面热电质谱法测定沉积物中的铅同位素组成[J].岩矿测试,2008,27(7):9-11. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS200801003.htm
[17] Cheng Z Q, Foland K A.Lead isotopes in tap water: Implications for Pb sources within a municipal water supply system[J].Applied Geochemistry, 2005, 20:353-365. doi: 10.1016/j.apgeochem.2004.09.003
[18] 黄斌.高灵敏度高精度铅同位素测定中的几个问题[J].质谱学报,1991,12(1):75-77. http://www.cnki.com.cn/Article/CJFDTOTAL-ZPXB199101011.htm
[19] 王林森,张利,贾旺鲁.大别-苏鲁超高压榴辉岩中Pb同位素分析的实验技术研究[J].地球科学--中国地质大学学报,2003,28(2):137-140. http://www.cnki.com.cn/Article/CJFDTOTAL-DQKX200302004.htm
[20] [21] 刘炳寰.质谱学方法与同位素分析[M].北京:科学出版社,1983.
[22] Cameron A E, Smith D H, Walker R L.Mass sper-ctrometry of nano-gramsiza samples of lead[J].Analytical Chemistry, 1969: 41(3):525-526. doi: 10.1021/ac60272a020
-




 下载:
下载:





