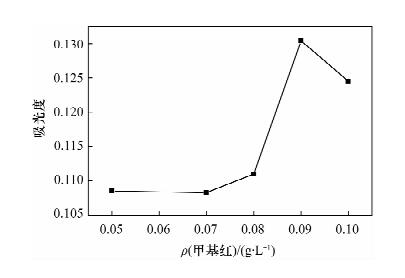
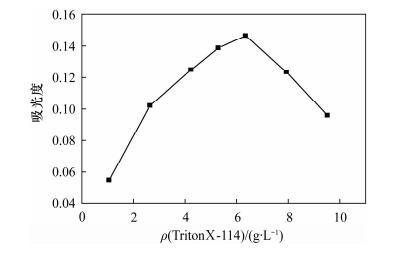
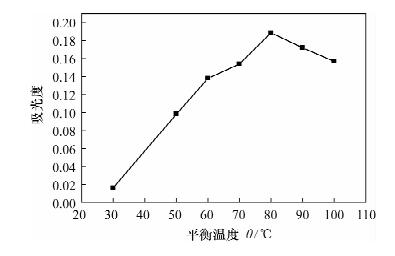
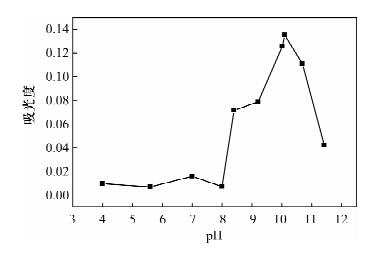
Determination of Available Cobalt in Soils by Flame Atomic Absorption Spectrometry with Cloud Point Extraction
-
摘要: 重金属的毒性和迁移性不仅取决于总量,而且取决于特殊的化学形态,其中有效态容易被植物吸收,对人体的危害更严重,因此准确测定土壤中的重金属有效态含量非常重要。但土壤中有效态金属的含量通常很低,而且干扰组分多,直接进行仪器分析测定比较困难。本文以0.1 mol/L盐酸为浸提剂,甲基红为螯合剂,Triton X-114为表面活性剂,建立了一种浊点萃取-火焰原子吸收光谱测定土壤中有效态钴的新方法,提高了测定的选择性和灵敏度。在最佳实验条件下,钴的线性范围为0.10~2.00 μg/mL,方法检出限为0.03 μg/mL,方法回收率为94.0%~104.0%,应用于分析0.5 μg/mL钴标准溶液,9次平行测定的相对标准偏差为3.3%。通过测定发现不同地区土壤中有效态钴占钴总量的比例差别很大,土壤中重金属有效态含量更能直观地传递出重金属在土壤中的迁移能力、存在状态以及危害程度等信息。Abstract: Toxicological studies have shown that available heavy metals is critical for risk assessment purpose since their transformations and potential toxicity depends, not only on total content, but also on the special chemical forms of the heavy metals. It is very difficult to measure heavy metals in soil by direct instrumental analysis due to the low contents of avalaible metals in soil samples and interference components. In the present paper, a novel procedure was proposed for the determination of available cobalt in soils by flame atomic absorption spectrometry with Cloud Point Extraction (CPE). In the proposed approach, 0.1 mol/L hydrochloric acid (HCl) was used as the extraction agent and methyl red as a chelating agent, with Triton X-114 being selected as the surfactant. The method improved the selectivity and sensitivity of determination. Under optimized conditions, the linear range is from 0.10 to 2.00 μg/mL, the detection limit is 0.03 μg/mL, the recoveries of the procedure ranged from 94.0% to 104.0%, and the relative standard deviation is 3.3% (ρ=0.5 μg/mL,n=9). It was found that the proportion of available cobalt is very different in soils from different areas. The contents of available heavy metals better reflected the information concerning the migration ability of heavy metals in soil, occurrence status and risk assessment.
-
Key words:
- soil /
- available cobalt /
- cloud point extraction /
- Flame Atomic Absorption Spectrometry
-

-
表 1 共存离子对钴测定的影响
Table 1. Effects of foreign ions on the preconcentration and determination of Co
共存离子 共存离子与Co的浓度比 回收率/% K+ 1000 106.0 Na+ 500 95.0 Mg2+ 50 90.0 Pb2+ 1000 108.0 Ca2+ 500 106.0 Cu2+ 300 108.0 Zn2+ 300 104.0 Ba2+ 500 101.0 Al3+ 100 110.0 Cr6+ 100 100.0 Ni2+ 10 92.0 Fe3+ 50 96.0 SO42- 5000 109.0 I- 5000 100.0 Br- 5000 102.0 NO3- 5000 96.0 Cl- 3000 100.0 Cd2+ 50 94.0 表 2 土壤样品中有效态钴的测定及回收率试验
Table 2. Determination of available cobalt in soil samples and recovery tests
实际土壤样品 w(Co)/(μg·mL-1) 回收率/% 加标量 平均测定值 郑东新区规划绿地 0 0.13 100.0 0.50 0.63 郑州高新区规划绿地 0 未检出 104.0 0.50 0.52 四川烟草地1 0 0.12 102.0 0.50 0.63 四川烟草地2 0 0.14 94.0 0.50 0.61 表 3 土壤样品中钴总量及有效态钴占钴总量的比例
Table 3. Total quantity of Co and available cobalt ratio in soil samples
实际土壤样品 w(Co)/(μg·g-1) 有效态钴占钴总量的比例/% 钴总量 有效态钴含量 郑东新区规划绿地 7.36 4.33 58.8 郑州高新区规划绿地 13.71 未检出 - 四川烟草地1 17.71 4.00 22.6 四川烟草地2 16.69 4.67 30.0 -
[1] Han H Y, Zhou J, Xu Y Y, Jia C L, Xia H. Determination of water-soluble and acid-soluble zinc in soils by flame atomic absorption spectrometry after cloud point extraction [J].Soil Science and Plant Analysis,2012,43(18):2389-2399. doi: 10.1080/00103624.2012.708072
[2] 查立新,马玲,刘文长,刘洪青,陈波,冯玲玲.振荡提取和超声提取用于土壤样品中元素形态分析[J].岩矿测试,2011,30(4):393-399. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS201104005.htm
[3] Han H Y, Xu Y Y, Zhang C.Determination of available cadmium and lead in soil by flame atomic absorption spectrometry after cloud point extraction [J].Soil Science and Plant Analysis,2011,42(14):1739-1751. doi: 10.1080/00103624.2011.584595
[4] 尹君,刘文菊,谢建治,肖崇彬.土壤中有效态镉、汞浸提剂和浸提条件研究[J].河北农业大学学报,2000,23(20):25-28. http://www.cnki.com.cn/Article/CJFDTOTAL-CULT200002007.htm
[5] 靳霞.土壤中重金属有效态的联合测定及其植物修复研究[D].临汾:山西师范大学,2012.
[6] 王畅,郭鹏然,陈杭亭,舒永红.土壤和沉积物中重金属生物可利用性的评估[J].岩矿测试,2009,28(2):108-112. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS200902008.htm
[7] HJ/T 166—2004,土壤环境监测技术规范[S].
[8] 李亮亮,张大庚,李天来,依艳丽,臧健,胡睿.土壤有效态重金属提取剂选择的研究[J].土壤,2008,40(5):819-823. http://www.cnki.com.cn/Article/CJFDTOTAL-TURA200805026.htm
[9] 熊礼明,鲁如坤. 土壤有效Cd浸提剂对Cd的浸提机制[J].环境化学,1992,11(3):41-47. http://www.cnki.com.cn/Article/CJFDTOTAL-HJHX199203005.htm
[10] 梁沛,李春香,秦永超,胡斌,江祖成.纳米二氧化钛分离富集和ICP-AES测定水样中Cr(Ⅵ)/Cr(Ⅲ) [J].分析科学学报,2000,16(4):300-303. http://www.cnki.com.cn/Article/CJFDTOTAL-FXKX200004008.htm
[11] Hosseini M S, Sarab A R R.Cr(Ⅵ)/Cr(Ⅲ) speciation in water samples by extractive separation using Amberlite CG 50 and final determination by FAAS [J].Inter-national Journal of Environmental Analytical Chemistry,2007,87(5):375-385. doi: 10.1080/03067310601068866
[12] Marques M J, Salvador A, Morales-Rubio A, de la Guardia M. Chromium speciation in liquid matrices:A survey of the literature [J].Fresenius Journal of Analytical Chemistry,2000,367(7):601-613. doi: 10.1007/s002160000422
[13] Balcerzak M, Swiecicka E.Determination of ruthenium and osmium in each other′s presence in chloride solutions by direct and third order derivative spectrophotometry [J].Talanta,1996,43:471-478. doi: 10.1016/0039-9140(95)01776-3
[14] Keith L H,Gron L U,Young J L. Green analytical methodologies [J].Chemical Reviews,2007,107(6):2695-2708. doi: 10.1021/cr068359e
[15] LY/T 1260—1999,森林土壤有效铜的测定[S].
[16] GB/T 17138—1997,土壤质量;铜、锌的测定;火焰原子吸收分光光度法[S].
-



 下载:
下载: