Study on the Phytodegradation of PAHs from Farmland Soil using Technique of Compound Specific Isotope Analysis
-
摘要:
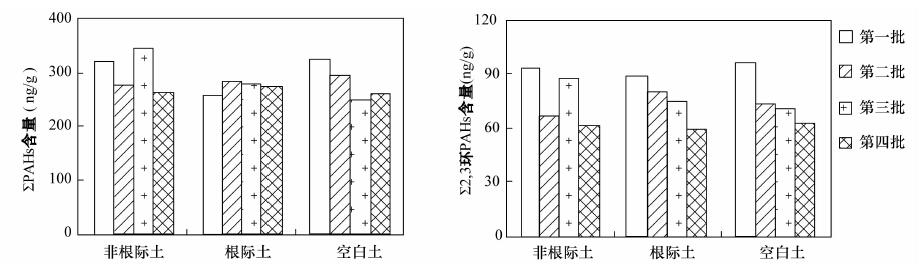
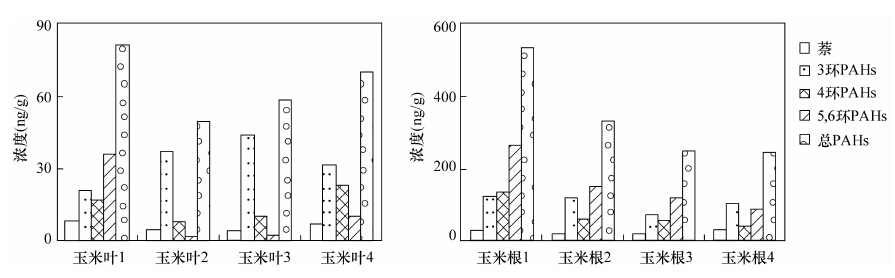
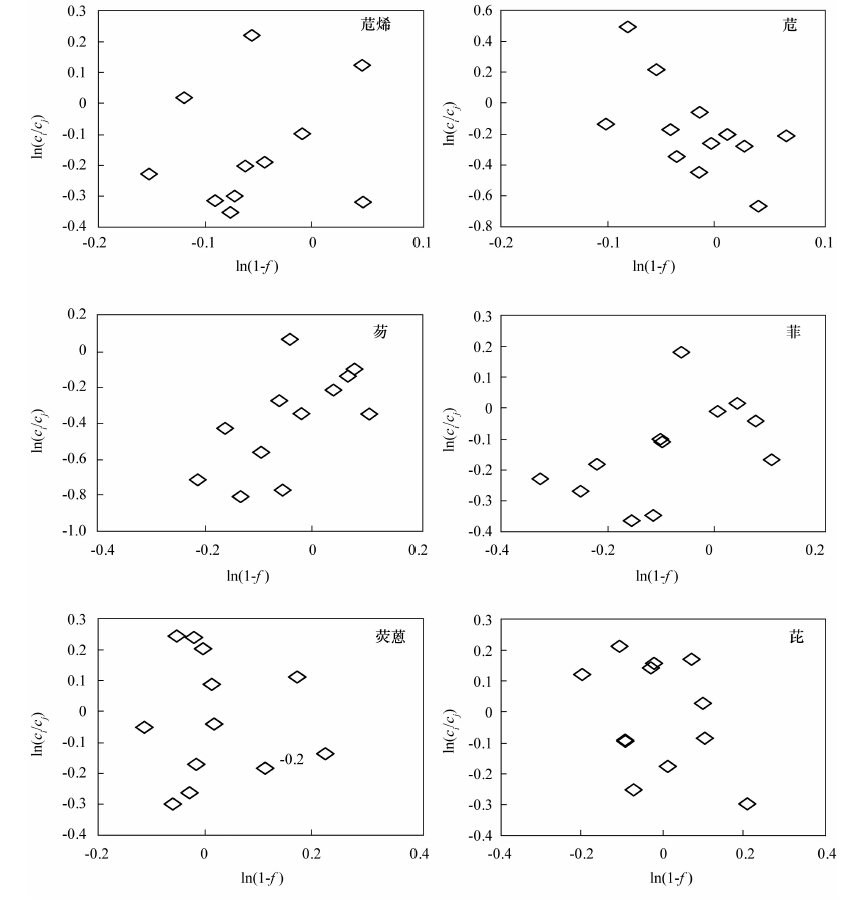
长期以来, 研究者在探讨土壤中多环芳烃(PAHs)的降解及修复过程中, 缺乏简便有效的手段对化合物的降解动态进行定量研究。前人尝试用投加实验、对比采用降解措施前后污染物的浓度变化、模型计算等方法研究PAHs的降解过程, 其结果常互相矛盾, 或不能真实反映复杂的实际环境。本文应用单体碳同位素技术对农田土壤中多环芳烃的植物降解过程进行定量表征, 采集了某地农田表土作为供试土壤, 选择玉米作为供试作物, 开展了作物对土壤中PAHs降解及消除过程的研究。气相色谱-质谱分析结果表明, 培养所用的玉米原始土及分4批收集的空白土、根际土、非根际土样品中16种PAHs的浓度总和(∑PAHs)平均分别为380.8 ng/g、(281.5±34.7) ng/g、(272.2±11.6) ng/g和(299.8±37.9) ng/g; 玉米生长期间, 各土壤样品的∑PAHs均比原始土壤有所下降, 但除3环化合物(苊烯、苊、芴、菲、蒽)外, 其他化合物并未随玉米的生长表现出显著趋势。与玉米根、叶倾向于富集低环PAHs化合物相对应, 可以判断植物对土壤中的低环化合物去除作用最为显著。各采样时期玉米根际土、非根际土和空白土壤样品中多环芳烃单体化合物的碳同位素分馏值(δ13C)在-34.31‰~-23.95‰之间, 且除芘外的其他化合物的δ13C值随时间呈现逐步变轻的趋势, 波动值位于-0.6‰~-9.0‰之间; 本文对于PAHs单体化合物, 尤其是4、5环化合物, 在玉米降解过程中的碳同位素分馏与浓度变化之间未发现明显关系。考虑3环以下的PAHs化合物更倾向于被降解和清除, 且其碳、氢同位素分馏情况更容易被观察到, 因此稳定同位素分析更有助于探明该类单体多环芳烃污染物在环境中的迁移、转化规律。
Abstract:For the research on degradation and remediation of soil polycyclic aromatic hydrocarbons (PAHs) pollutants, researchers have been trying to find an effective method to quantize the dynamic processes. Many approaches have been designed, such as spiked experiment, concentrations comparison before and after the degradation, and modeling calculations. However, all of this culminates in contradictory results and lack of corroboration of the actual environment. Compound Specific Isotope Analysis (CSIA) technique is used in this study to investigate the phytodegradation process of soil PAHs with maize as the testing plant. Total concentrations of 16 PAHs in the bulk soil, rhizosphere soil and in the controlled soil samples were (299.8±37.9) ng/g, (272.2±11.6) ng/g, and (281.5±34.7) ng/g, respectively, all much lower than the original cultivating soil (380.8 ng/g). The soil PAHs concentrations did not show significant decline tendency during the maize growing period, except for 3-ring PAH compounds which was consistent with the accumulation effect of the 3-ring PAHs in maize roots and leaves. The δ13C value of 6 PAH compounds ranged from -34.31‰ to -23.95‰ with fluctuations between -0.6‰--9.0‰, and all δ13C values turned lighter with maize plants growing except that of Pyrene. No significant correlation between δ13C and the concentration of 4-, 5-ring PAHs was observed during the maize cultivating process. Most research indicates that PAHs and other macromolecular compounds strongly resist phytodegradation, which illustrates less obvious carbon isotope fractionation. PAH compounds below 3 rings would degrade more easily and the isotopes fractionate more significantly than those of other macromolecular compounds, thus maing the technique of CSIA more advantageous for understanding the transportation pathway of these compounds.
-

-
表 1 玉米室内培养方案及采样说明
Table 1. The indoor cultivate and sample programme of maize plants
采样
批次采样
时间样品干重(g) 玉米叶 玉米根 根际土 非根际土 对照空白土 第一批 第7天 7.33 3.13 10 10 10 第二批 第12天 7.41 5.29 10 10 10 第三批 第16天 9.84 6.69 10 10 10 第四批 第21天 7.57 4.95 10 10 10 表 2 不同生长时期土壤中PAHs浓度及有机碳含量
Table 2. Concentrations of PAHs and TOC in soil samples during 4 sample periods
PAHs化合物 非根际土(ng/g,干重) 根际土(ng/g,干重) 空白土(ng/g,干重) 原始土
(ng/g,干重)第一批 第二批 第三批 第四批 第一批 第二批 第三批 第四批 第一批 第二批 第三批 第四批 萘 21.54 9.93 17.97 13.10 16.27 10.63 16.97 6.13 21.50 14.92 13.50 11.47 14.92 苊烯 2.23 1.62 2.02 1.65 1.95 2.21 1.94 1.61 2.02 2.25 1.79 1.58 1.56 苊 3.93 nd 3.31 2.51 3.70 2.99 nd 2.62 4.12 3.32 3.13 2.56 2.26 芴 7.56 6.11 5.35 3.49 10.51 9.56 6.84 4.68 8.69 5.34 5.75 4.07 4.66 菲 50.59 42.82 51.47 35.70 50.41 48.41 41.80 38.50 52.76 41.40 41.03 37.14 19.64 蒽 7.18 6.36 7.64 5.25 6.04 6.88 7.51 5.82 7.22 6.37 5.96 5.91 5.23 荧蒽 31.26 27.37 35.10 26.11 23.93 30.54 29.50 29.45 32.16 28.71 22.15 24.29 23.52 芘 40.50 36.94 45.75 33.91 30.99 35.77 36.79 36.78 40.43 37.56 29.14 34.14 36.52 苯并[a]蒽 28.21 26.09 32.31 25.65 21.00 25.53 25.78 27.10 29.35 27.72 23.05 24.87 30.78 䓛 32.49 29.63 36.78 29.30 24.24 28.44 29.17 30.86 33.43 31.23 27.27 29.67 37.69 印并[123, cd]芘 2.07 5.28 2.06 1.55 1.26 1.52 4.08 1.53 1.97 1.54 1.70 1.20 42.80 苯并[a]芘 6.37 nd 6.93 5.16 4.51 5.33 nd 5.86 6.05 6.16 5.15 5.49 13.77 苯并[ghi]苝 20.92 21.24 23.27 18.78 14.42 18.13 21.00 19.92 20.56 21.07 16.91 18.81 81.22 苯并[b]荧蒽 7.79 8.30 9.05 7.57 5.53 7.25 7.78 8.01 8.02 8.24 6.05 6.82 84.64 苯并[k]荧蒽 31.98 29.66 36.81 29.16 23.30 27.88 27.25 30.96 31.85 32.12 26.45 28.86 31.34 苯并[a, h]蒽 24.21 23.67 27.73 23.04 17.64 21.15 21.21 23.72 25.09 25.48 19.86 21.93 50.30 ∑PAHs 318.80 275.00 343.52 261.94 255.71 282.23 277.64 273.54 325.22 293.43 248.89 258.80 480.83 2, 3环PAHs 93.03 66.84 87.77 61.70 88.89 80.69 75.06 59.35 96.32 73.60 71.16 62.72 48.27 4, 5, 6环PAHs 225.78 208.17 255.79 200.25 166.83 201.54 202.58 214.19 228.91 219.84 177.74 196.08 432.59 总有机碳(%) 0.82 0.86 0.87 0.83 0.94 1.01 0.97 0.87 nd 0.94 0.91 0.84 0.88 注:“nd”表示未检出。 表 3 16种PAHs化合物的基本理化参数
Table 3. The physical-chemical parameters of 16 PAHs compounds
PAHs
化合物logKow logH logKoa PAHs
化合物logKow logH logKoa 萘 3.37 1.6335 5.1308 苯并[a]蒽 5.91 -0.991 10.296 苊烯 4.00 1.0626 6.3317 䓛 5.86 -0.975 10.229 苊 3.92 1.3802 5.934 苯并[b]荧蒽 5.80 -1.268 10.462 芴 4.18 0.9294 6.6448 苯并[k]荧蒽 6.00 -0.955 10.349 菲 4.57 0.6021 7.3622 苯并[a]芘 6.04 -2.046 11.480 蒽 4.54 0.7782 7.1561 茚并[123-cd]芘 6.50 - - 荧蒽 5.22 -0.181 8.7954 二苯并[a, h]蒽 6.75 -2.155 12.299 芘 5.18 0.0414 8.5329 苯并[ghi]苝 6.50 -3.000 12.894 表 4 玉米培养过程中土壤PAHs的碳同位素特征
Table 4. The fractionation of PAHs carbon isotope during the maize cultivating process
样品编号 δ13C(‰) 苊烯 苊 芴 菲 荧蒽 芘 非根际土1 -26.47 -29.52 -29.97 -29.68 -33.85 -26.52 非根际土2 -24.60 -27.94 -28.38 -25.53 -24.82 -30.20 非根际土3 -26.87 -31.20 -25.70 -28.08 -26.94 -34.42 非根际土4 -29.37 -30.14 -32.16 -34.31 -29.35 -25.98 根际土1 -26.42 -29.08 -28.14 -29.65 -28.25 -30.17 根际土2 -24.58 -26.46 -24.97 -26.69 -29.08 -31.29 根际土4 -28.20 -30.53 -33.45 -39.63 -28.40 -27.27 空白土2 -26.21 -29.68 -26.15 -30.92 -28.91 -23.95 空白土3 -32.28 -30.27 -27.80 -30.79 -30.07 -26.79 空白土4 -29.27 -29.85 -28.59 -34.89 -29.57 -27.60 -
[1] [2] [3] [4] [5] [6] Kim M K. Stable Carbon Isotope Ratio of Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Validation of Isolation and Stable Carbon Isotope Analysis Methods[D]. Texas A & M University, 2004.
[7] [8] [9] USEPA 8310. Polynuclear Aromatic Hydrocarbons [S]. US Environmental Protection Agency, 1986.
[10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] -



 下载:
下载: