Mechanism Study of Cadmium(Ⅱ) Adsorption on Thiol-Modified Montmorillonite
-
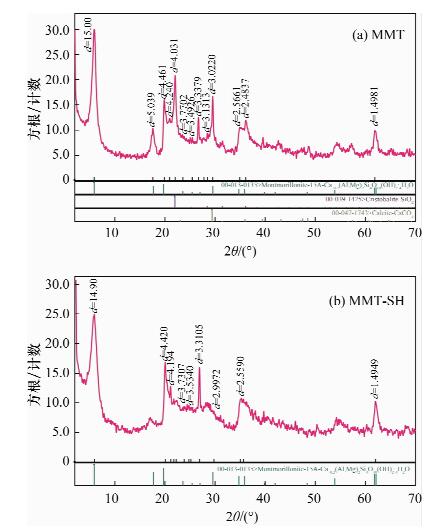
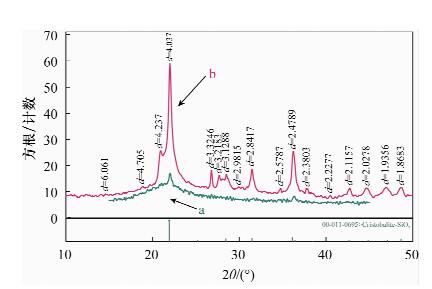
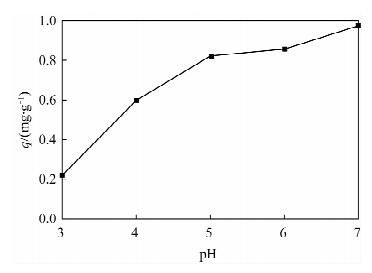
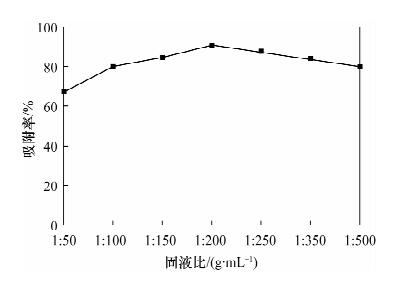
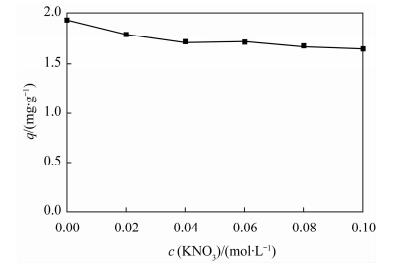
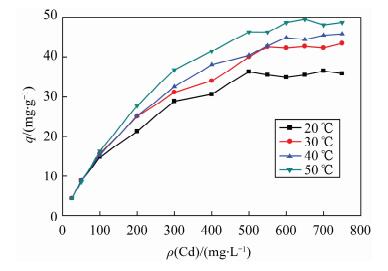
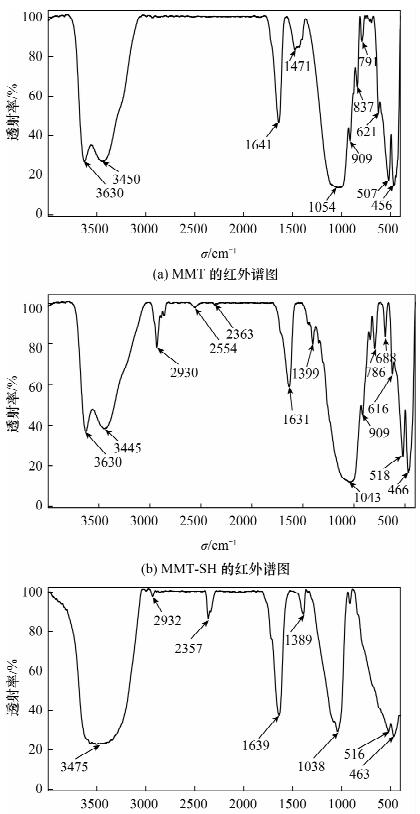
摘要: 蒙脱石对镉有良好的吸附性,但吸附力较弱,巯基改性蒙脱石可增强对镉的吸附,而关于蒙脱石的巯基改性及改性后对镉的吸附机理研究少见报道。本文以(3-巯丙基)三甲氧基硅烷作为改性剂,采用简单的溶液法对蒙脱石原土(简称MMT)进行巯基改性,并探讨了改性蒙脱石(简称MMT-SH)对镉的吸附机理。红外和X射线衍射表征显示MMT被成功接上巯基。吸附条件实验表明,MMT-SH对Cd(Ⅱ)的吸附效果显著优于原土,对Cd(Ⅱ)的饱和吸附容量是原土的39倍;吸附容量受pH值影响大,受离子强度的影响较大;MMT-SH对Cd(Ⅱ)的吸附作用除了静电吸附、离子交换吸附、羟基配位吸附,还主要存在巯基配位吸附。热力学及动力学实验表明,MMT-SH吸附Cd(Ⅱ)的反应符合Langmiur等温模型和Lagergren二级动力学方程,是易于发生的化学反应;吸附过程的热力学参数(ΔH、ΔG、ΔS)表明此吸附是一个自发的吸热过程。Abstract: Montmorillonite (MMT) is a good sorbent of cadmium, but the adsorbability is weak. Thiol-Modified Montmorillonite could increase the adsorption of cadmium, but the studies about thiol-modified montmorillonite (MMT-SH) and the adsorption mechanism of cadmium on MMT-SH are rarely reported. In this study, the MMT-SH was prepared through modifying natural MMT with 3-mercapto-propyl-trimethoxysilane and the adsorption mechanism of cadmium on MMT-SH was investigated. The characteristic results of FT-IR and XRD indicated the successful tethering of the thiol group on MMT. The experimental results of cadmium adsorption on MMT-SH showed that the adsorption efficiency of cadmium on MMT-SH was much better than that on natural MMT, which had been increased 39 times. Adsorption efficiency was influenced by ionic strength and pH value of the system. Except for electrostatic adsorption, ion-exchange and hydroxyl coordination, the thiol coordination was one of the main reactions in adsorption of cadmium on MMT-SH. The adsorption behavior of cadmium on MMT-SH fitted well with the Langmuir adsorption isotherm and Lagergren-second-order kinetic model which indicates that the adsorption is a chemical process. The thermodynamic parameters (ΔH, ΔGandΔS) show that the adsorption of cadmium on MMT-SH is a spontaneous endothermic process.
-
Key words:
- montmorillonite /
- thiol-modified /
- cadmium /
- adsorption mechanism
-

-
表 1 MMT-SH对Cd(Ⅱ)的吸附等温方程拟合结果
Table 1. Adsorption isotherm equations for cadmium adsorption on MMT-SH
等温模型 温度/℃ KL Qm/(mg·g-1) 1/n AT E(bT)/(kJ·mol-1) R2 Langmuir方程 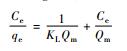
20 0.025 38.76 - - - 0.9931 30 0.022 46.51 - - - 0.9910 40 0.020 49.50 - - - 0.9938 50 0.025 52.91 - - - 0.9975 Freundlich方程 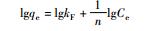
20 5.234 - 0.3192 - - 0.9816 30 5.237 - 0.3504 - - 0.9882 40 5.134 - 0.3633 - - 0.9916 50 3.587 - 0.4616 - - 0.9753 Tempkin方程 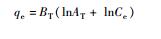
20 - - - 0.5625 42.71 0.9752 30 - - - 0.5124 36.63 0.9797 40 - - - 0.4333 34.74 0.9822 50 - - - 0.4504 32.51 0.9835 D-R方程 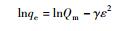
20 - 76.86 - - 11.08 0.9790 30 - 96.62 - - 11.12 0.9885 40 - 107.5 - - 11.17 0.9796 50 - 125.3 - - 11.34 0.9889 注:Ce为平衡浓度,mg/L;qe为平衡吸附量,mg/g;KL为Langmuir吸附常数;Qm为饱和吸附量,mg/g;C0为初始浓度,mg/L;n为Freudlich常数;BT为Temkin自由能活度系数;AT为Tempkin吸附potential,L/mg;bT为Temkin吸附热,kJ/mol;γ为D-R吸附自由能活度系数,mol2/kJ2;ε为Polanyi系数;E为吸附自由能,kJ/mol。 表 2 MMT-SH吸附Cd(Ⅱ)的热力学变化
Table 2. Adsorption thermodynamics data of Cd on MMT-SH
T/K ΔH/(kJ·mol-1) ΔG/(kJ·mol-1) ΔS/(J·mol-1·K) 293 40.19 -13.71 184.2 303 40.19 -15.07 184.2 313 40.19 -16.58 184.2 323 40.19 -17.29 184.2 表 3 MMT-SH对Cd(Ⅱ)的吸附动力学拟合结果
Table 3. Kinetics results for cadmium adsorption on MMT-SH at initial concentration
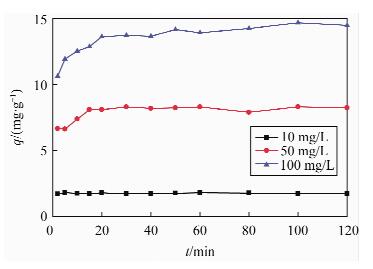
吸附方程 Cd(Ⅱ)浓度ρ/(mg·L-1) a b k qe/(mg·g-1) 相关系数 Lagergren一级方程 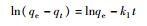
10 - - -4×10-5 1.813 0.0067 50 - - 0.0011 9.182 0.367 100 - - 0.0016 16.26 0.6687 Lagergren二级方程t 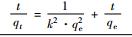
10 - - 2.9404 1.750 0.9997 50 - - 0.1915 8.278 0.9993 100 - - 0.03764 14.68 0.9995 Elovich方程 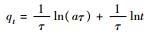
10 1.7587 0.0006 - - 0.0005 50 6.4572 0.4314 - - 0.7483 100 10.355 0.9243 - - 0.9571 抛物线扩散方程 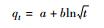
10 1.763 -0.0004 - - 0.0016 50 6.9681 0.1503 - - 0.5444 100 11.298 0.3473 - - 0.8101 Freundlich修正式 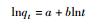
10 0.5643 0.0004 - - 0.0007 50 1.8704 0.0579 - - 0.7469 100 0.0726 0.0726 - - 0.9446 注:qe为平衡吸附量,mg/g;qt为t时刻的吸附量,mg/g;k1为一级吸附速率常数,min-1;t为时间,min;k2为二级吸附速率反应常数,g/(mg·min);a为化学吸附速率常数,τ与表面覆盖率有关的常数;抛物线扩散方程中b用来解释离子的表观速度;Freundlich修正式中b 为速率常数。 -
[1] 梁彦秋,刘婷婷,铁梅,邓斌,孙鹏,藏树良.镉污染土壤中镉的形态分析及植物修复技术研究[J].环境科学与技术,2007,2(3): 57-58. http://www.cnki.com.cn/Article/CJFDTOTAL-FJKS200702020.htm
[2] 鞠建英,申东炫.膨润土在工程中的开发与应用[M].北京: 中国建材工业出版社,2003: 1-2.
[3] Balomenou G, Stathi P, Enotiadis A, Gournis D, Deligian-nakis Y. Physicochemical study of amino-functionalized organosilicon cubes intercalated in montmorillonite clay: H-binding and metal uptake [J]. Journal of Colloid and Interface Science,2008,325: 74-83. doi: 10.1016/j.jcis.2008.04.072
[4] 原金海,邓利均.改性膨润土的制备及其对Pb2+的吸附性能研究[J].功能材料,2011,42(6): 980-984.
[5] 李增新,王彤,黄海兰,张道来,孟韵.壳聚糖改性膨润土修复土壤镉污染的研究[J].土壤通报,2009,40(1): 176-178. http://www.cnki.com.cn/Article/CJFDTOTAL-TRTB200901047.htm
[6] 朱霞萍.酸雨条件下珠江三角洲土壤中镉砷的迁移规律研究[D].成都: 成都理工大学, 2008.
[7] 李虎杰,刘爱平,易发成,白萍.膨润土对Cd2+的吸附作用及影响因素[J].中国矿业,2004(11): 79-81. doi: 10.3969/j.issn.1004-4051.2004.11.024
[8] 曾江萍.镉在膨润土中的吸附和解吸行为研究[D].成都: 成都理工大学,2008.
[9] 朱霞萍,白德奎,李锡坤,曾江萍,曹三勇.镉在蒙脱石等黏土矿物上的吸附行为研究[J].岩石矿物学杂志,2009,28(6): 643-648.
[10] Huang R H, Wang B, Yang B C, Zheng D S, Zhang Z Q.Equilibrium, kinetic and thermodynamic studies of adsorption of Cd(Ⅱ) from aqueous solution onto HACC-bentonite [J]. Desalination,2011,280: 297-304. doi: 10.1016/j.desal.2011.07.033
[11] 周建兵,吴平霄,朱能武,党志.十二烷基磺酸钠(SDS)改性蒙脱石对Cu2+、Cd2+的吸附研究[J].环境科学学报,2010,30(1): 88-96.
[12] 王毅,王艺,王恩德.改性蒙脱石吸附Pb2+、Hg2+的实验研究[J].岩石矿物学杂志,2001,20(4): 565-567.
[13] 张一平.膨润土的巯基功能化研究[J].浙江教育学院学报,2007(1): 35-37. http://www.cnki.com.cn/Article/CJFDTOTAL-LJYX201205008.htm
[14] Mercier L, Preparation C D. Characterization, and application as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite [J]. Environmental Science & Technology , 1995, 29: 1318-1323.
[15] Malferrari D, Brigatti M F, Laurora A, Pini S, Medici L. Sorption kinetics and chemical forms of Cd (Ⅱ) sorbed by thiol-functionalized 2∶1 clay minerals [J]. Journal of Hazardous Materials, 2007,143: 73-81. doi: 10.1016/j.jhazmat.2006.08.069
[16] Liang X F,Xu Y M,Sun G H,Wang L,Sun Y,Qin X.Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+and Cd2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2009,349: 61-68.
[17] 李虎杰.盐亭膨润土的物化性能及对Pb2+、Cd2+的吸附研究[J].中国矿业,2006,15(5): 76-79.
[18] Ijagbemi C O, Baek M H, Kim D S.Adsorptive perfor-mance of un-calcined sodium exchanged and acid modified montmorillonite for Ni2+removal: Equilibrium, kinetics, thermodynamics and regeneration studies [J]. Journal of Hazardous Materials, 2010,174: 746-755. doi: 10.1016/j.jhazmat.2009.09.115
[19] Eren E.Removal of lead ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated forms [J]. Journal of Hazardous Materials,2009,165: 63-70. doi: 10.1016/j.jhazmat.2008.09.066
[20] Grawal A A, Sahu K K. Kinetic and isotherm studies of cadmium adsorption on manganese nodule residue [J]. Journal of Hazardous Materials,2006,137: 915-924. doi: 10.1016/j.jhazmat.2006.03.039
[21] Yang S T, Zhao D L, Zhang H, Lu S S, Chen L, Yu X J. Impact of environmental conditions on the sorption behavior of Pb(Ⅱ) in Na-bentonite suspensions [J]. Journal of Hazardous Materials,2010,183: 632-640. doi: 10.1016/j.jhazmat.2010.07.072
[22] von Open B, Kordel W, Klein W.Sorption of nonpolar and polar compounds to soils: Processes, measurement and experience with the applicability of the modified OECD-guideline [J]. Chemosphere,1991,22: 285-304. doi: 10.1016/0045-6535(91)90318-8
[23] 李朝丽,周立祥.黄棕壤不同粒级组分对镉的吸附动力学与热力学研究[J].环境科学,2008,29(5): 1406-1411. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ200805049.htm
-



 下载:
下载: