Characteristics, controlling factors and effects on human health of groundwater chemical evolution in Wenzhou Plain, lower Oujiang River catchment
-
摘要:
研究目的 滨海地带地下水化学演化特征及其控制因素研究对沿海城市地下水资源可持续利用具有重要意义。
研究方法 在野外调查取样和历史资料综合分析的基础上,运用水化学图解、离子比例关系、多元统计分析及环境同位素方法,系统分析了温州平原地下水化学演化特征,探讨了影响地下水化学演化的主要控制因素。
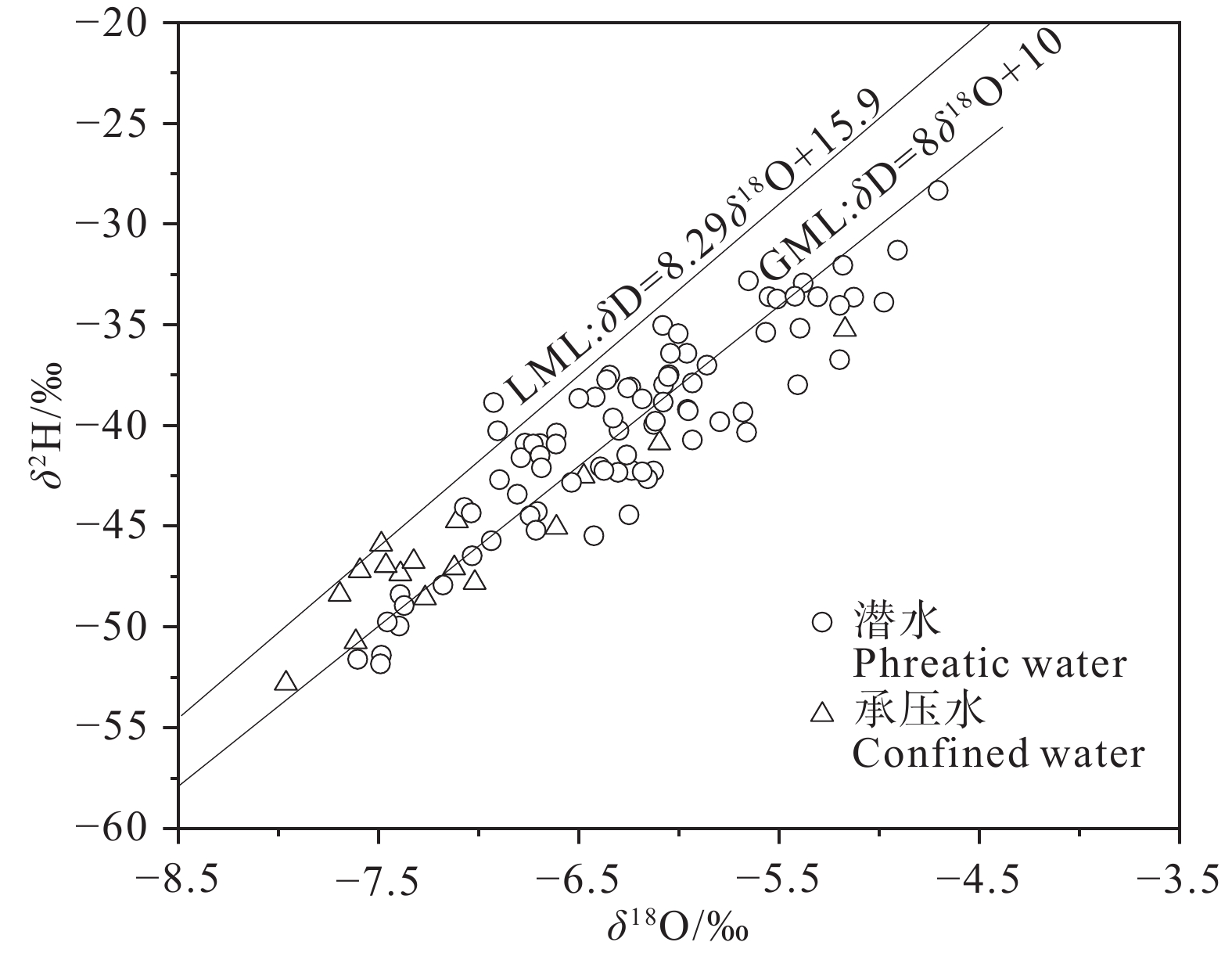
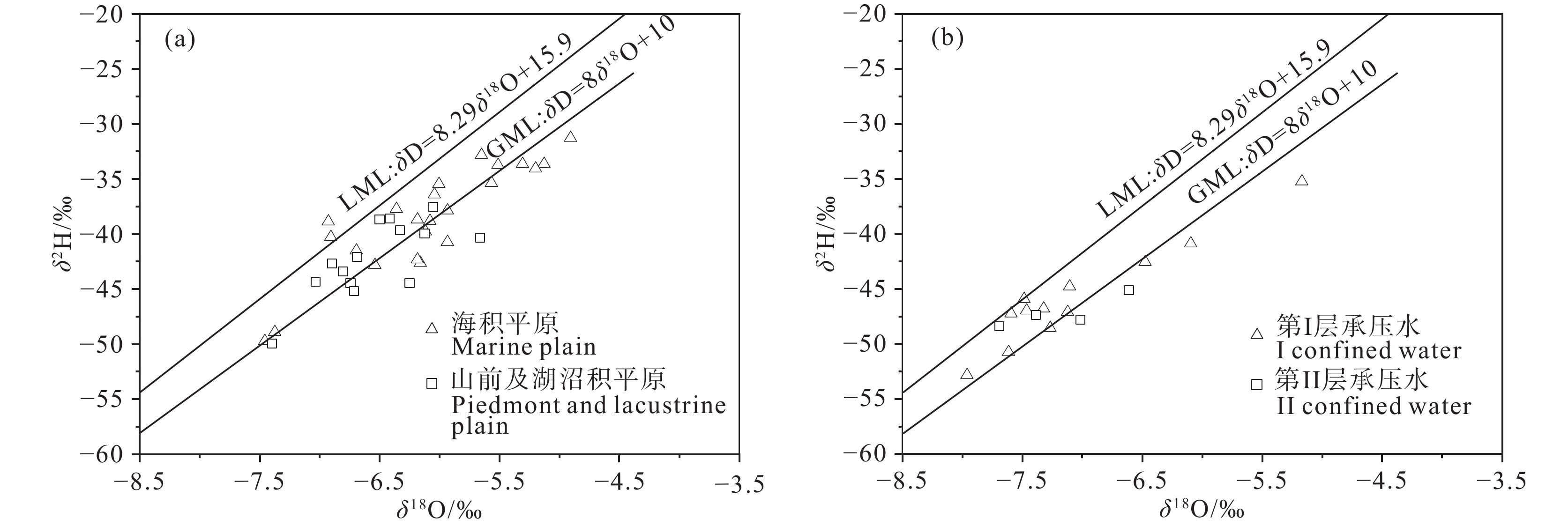
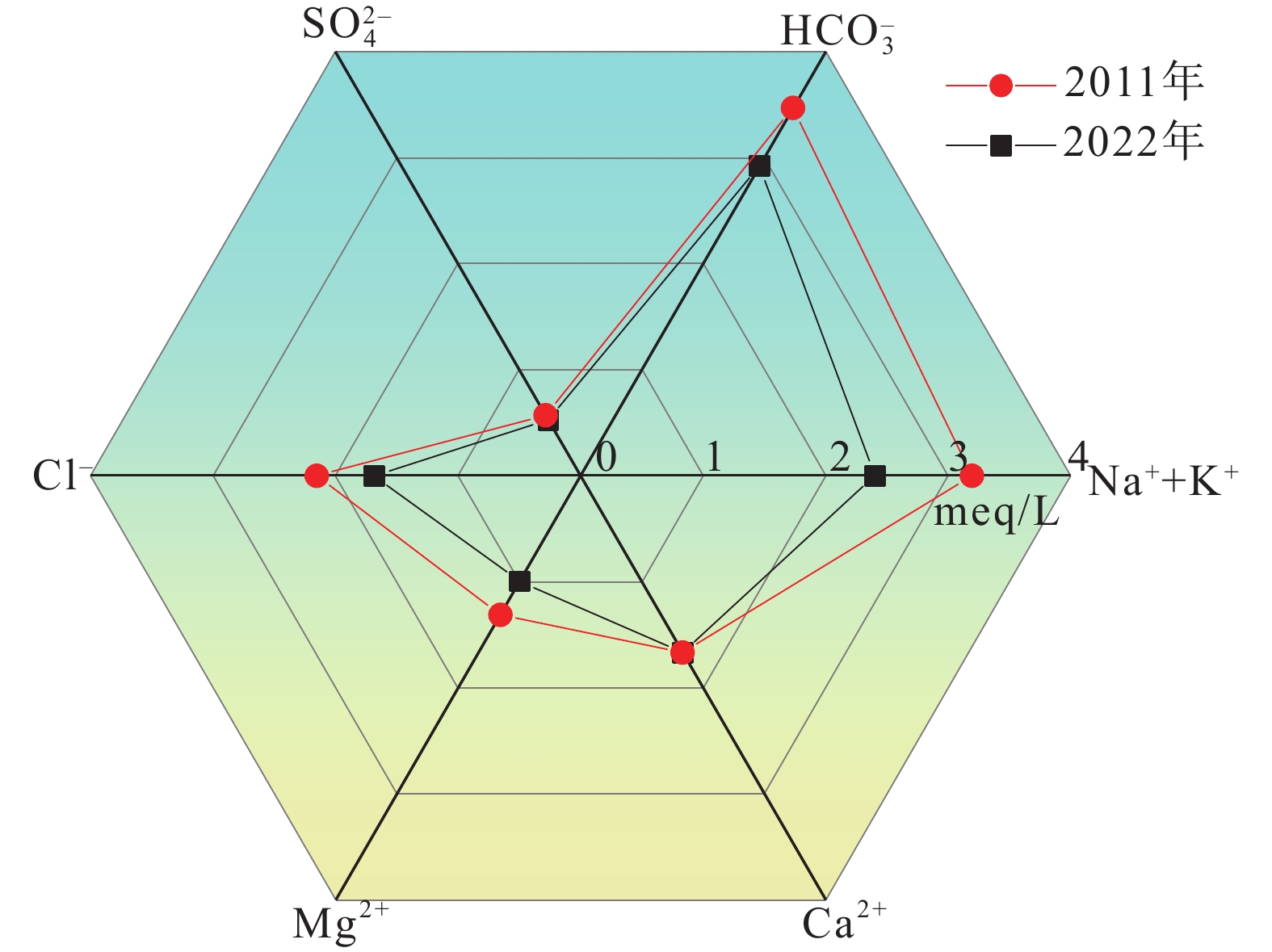
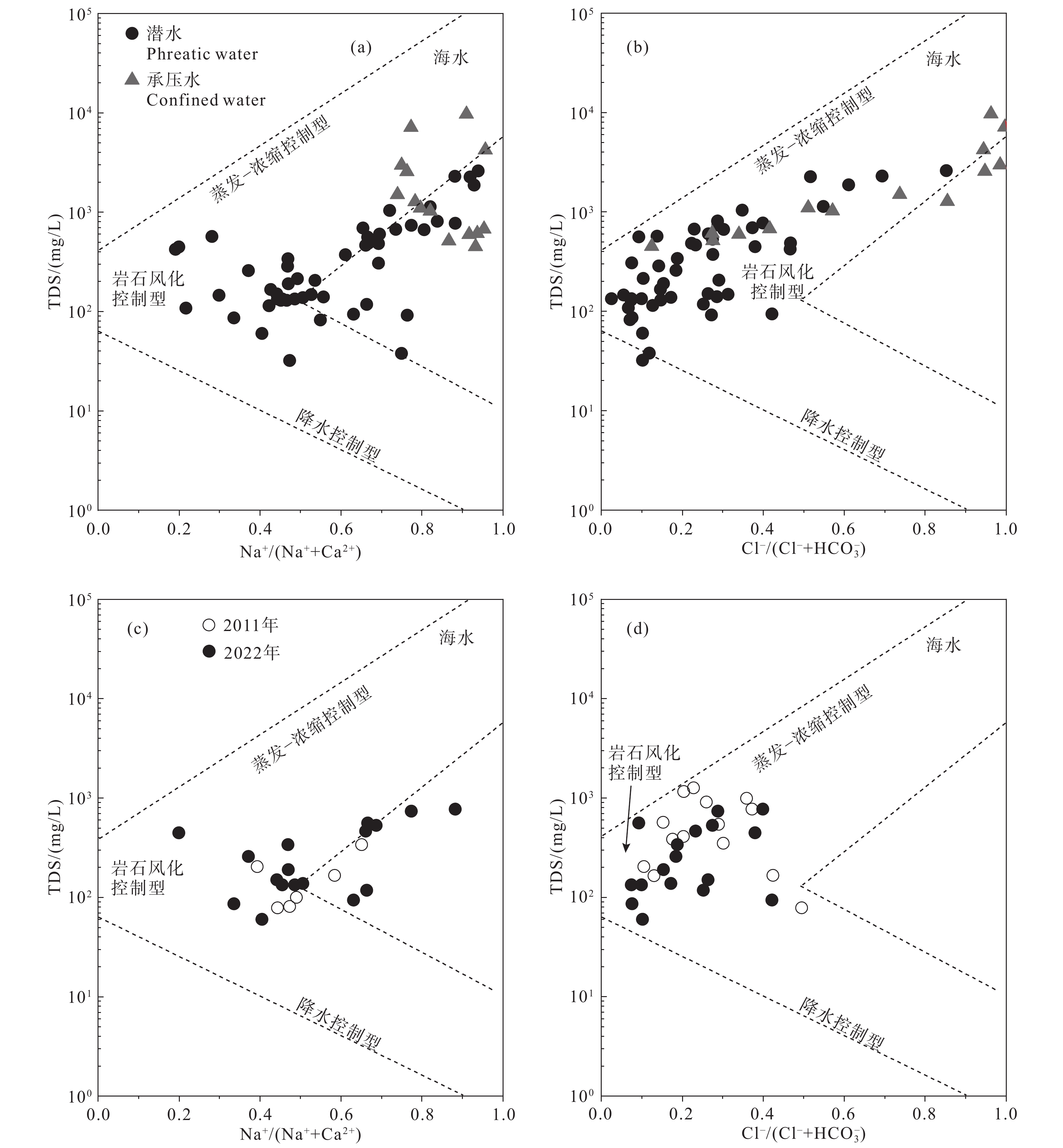
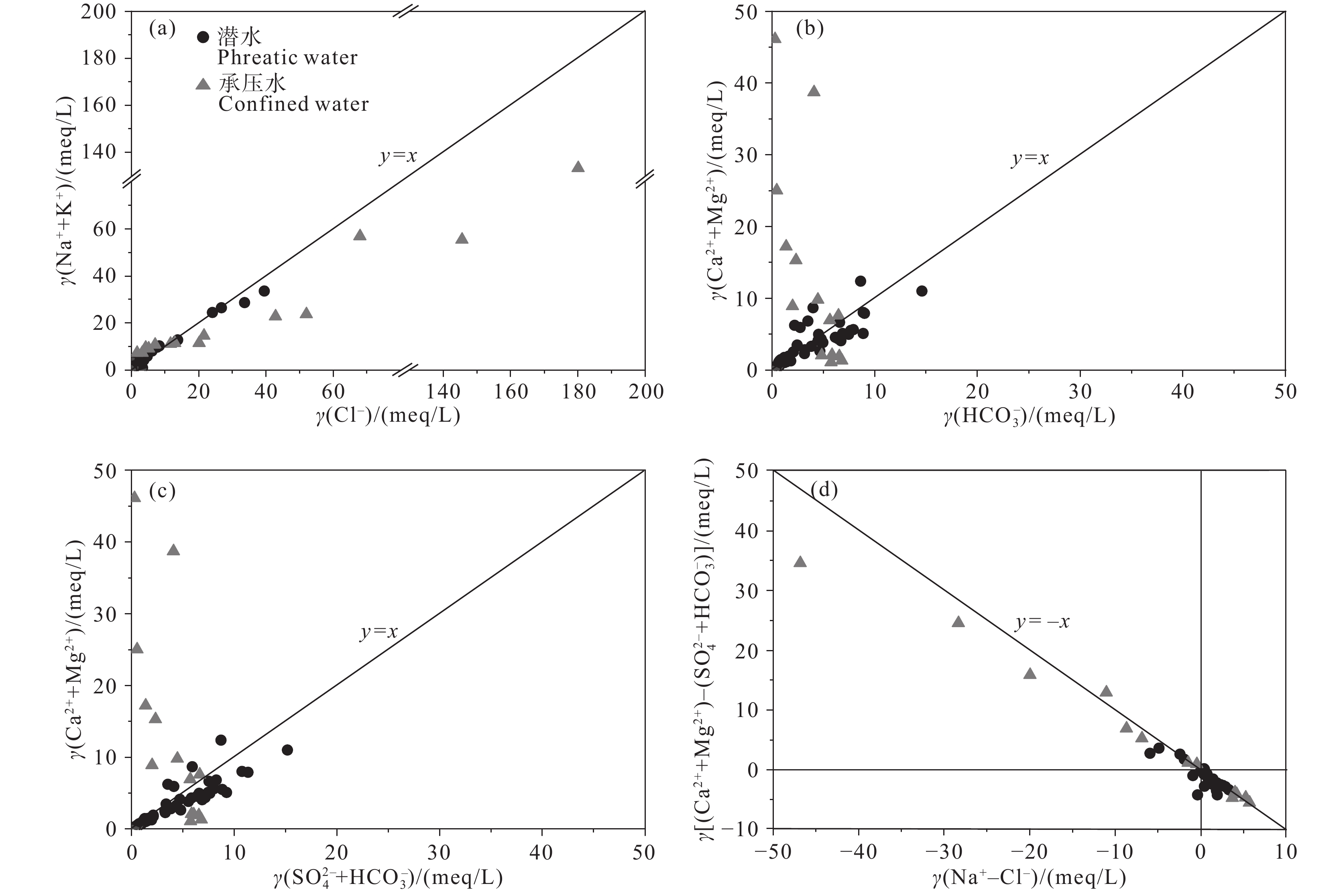
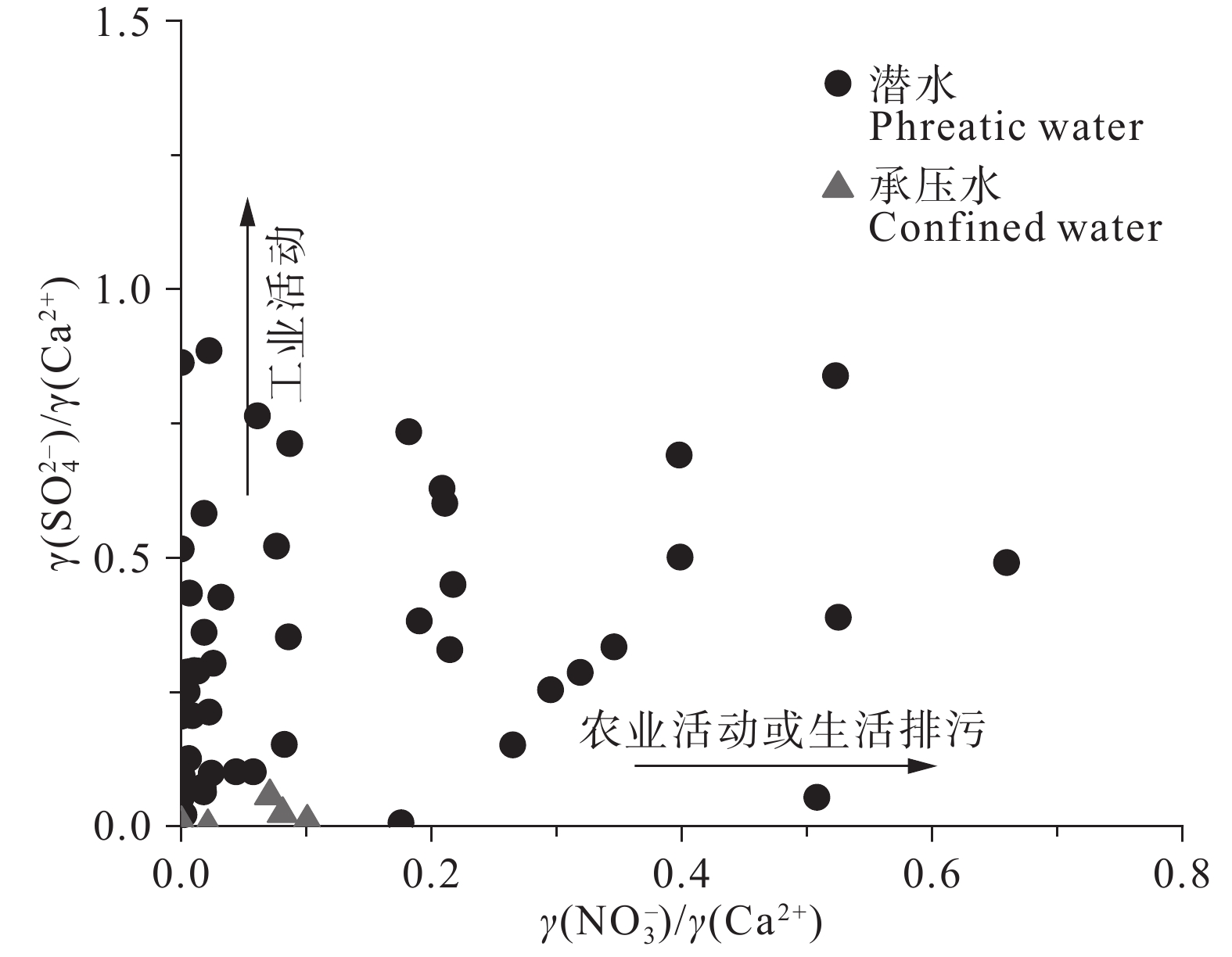
研究结果 (1)温州平原潜水以淡水为主,HCO3–、Na+、Ca2+占主导地位,承压水以微咸水和咸水为主,Cl–、Na+占绝对优势;(2)从山前到海积平原,研究区潜水由低矿化度的HCO3型水向较高矿化度的Cl型水转变,主要受天然水化学作用控制,人类活动使局部地区地下水化学出现异常;(3)十年尺度上,潜水主要组分含量存在一定程度下降,水化学类型向Cl–比重减少、HCO3–比重增加的方向演化;(4)水岩相互作用、海水作用、氧化还原环境等自然因素和工农业生产、生活排污等人为因素是温州平原地下水化学演化的主要控制因素。
结论 地下水健康风险评价结果显示,区内地下水存在一定的潜在非致癌风险,潜水的非致癌风险小于承压水,饮水途径摄入是危害人体的主要途径,相同环境下儿童的非致癌风险高于成人。因此,有必要对存在健康风险的地下水进行长期监测,加强这类地区的地下水资源管理和污染防治。
Abstract:This paper is the result of hydrogeological survey engineering.
Objective The study on the characteristics of groundwater chemical evolution and its control factors in coastal zones is of great significance for the sustainable utilization of groundwater resources in coastal cities.
Methods On the basis of field investigation and comprehensive analysis of historical data, the characteristics of groundwater chemical evolution in Wenzhou Plain were systematically analyzed by using hydrochemical diagram, ion proportional relationship, multivariate statistical analysis and environmental isotope methods, and the main controlling factors affecting groundwater chemical evolution were discussed.
Results (1) Phreatic water in Wenzhou Plain was dominated by fresh water, and HCO3–, Na+ and Ca2+ were the predominant ions. However, confined water is brackish and salt water, and Cl– and Na+ were absolutely dominant ions. (2) From the mountain front to the marine deposition plain, the transition from low−salinity HCO3 type water to high−salinity Cl type water in the study area is mainly controlled by natural processes, and human activities caused abnormalities of local groundwater chemistry. (3) On the ten−year scale, the content of the main components of phreatic water decreased to a certain extent, and the hydrochemical type evolved in the direction of the decrease of Cl– and the increase of HCO3–. (4) Natural factors such as water−rock interaction, seawater interaction, redox environment and human factors such as industrial and agricultural production and domestic sewage are the main controlling factors of groundwater chemical evolution in Wenzhou Plain.
Conclusions The results of groundwater health risk assessment show that certain potential non−carcinogenic risks of groundwater exist in the area, the non−carcinogenic risk of phreatic water is less than that of confined water, drinking water intake is the main way to harm human body, and the non−carcinogenic risk of children in the same environment is higher than that of adults. Therefore, it is necessary to conduct long−term monitoring of groundwater with health risks and strengthen groundwater resource management and pollution prevention in such areas.
-

-
图 2 研究区水文地质剖面示意图(修改自李银法, 1985
4 )Figure 2.
表 1 地下水水化学参数特征值统计
Table 1. Statistics of hydrochemical parameters of groundwater
类型 统计指标 最小值 最大值 平均值 标准差 变异系数 标准值 超标率/% 潜水(n=50) pH 6.36 8.81 7.19 0.5 0.08 6.5~8.5 8 K+ 1.32 122.00 16.98 20.2 1.19 Ca2+ 1.84 107.45 37.85 25.6 0.68 Na+ 2.48 772.00 110.22 174.9 1.59 200 14 Mg2+ 1.09 100.06 18.46 23.2 1.25 Cl– 2.16 1401.80 154.93 300.0 1.94 250 14 HCO3– 19.00 889.87 218.77 193.1 0.88 SO42– 1.01 228.27 32.10 38.7 1.21 250 0 NO3– <0.02 77.28 7.93 12.3 1.55 88.57 0 NH4+ <0.013 17.41 1.04 2.8 2.72 0.64 24 Mn <0.004 3.45 0.66 1.0 1.49 0.1 50 Br– <0.06 4.71 0.38 0.8 2.15 TDS 32 2598.00 492.68 586.7 1.19 1000 12 承压水(n=14) pH 6.54 7.93 7.41 0.41 0.06 6.5~8.5 0 K+ 2.87 40.97 12.66 10.71 0.85 Ca2+ 12.18 375.73 105.86 109.52 1.03 Na+ 168.70 3066.00 632.59 767.38 1.21 200 86 Mg2+ 5.27 328.05 94.18 101.85 1.08 Cl– 59.01 6393.59 1460.49 1906.73 1.31 250 64 HCO3– 17.37 414.04 247.76 136.65 0.55 SO42– <0.1 57.13 5.38 14.53 2.70 250 0 NO3– <0.02 19.67 4.91 6.56 1.34 88.57 0 NH4+ 0.04 14.99 5.20 4.32 0.83 0.64 93 Mn 0.01 2.13 0.56 0.67 1.19 0.1 79 Br– <0.06 8.84 2.18 2.54 1.16 TDS 450.00 9700.00 2451.43 2695.07 1.10 1000 64 注:pH无量纲,其余指标单位为mg/L;标准值为《地下水质量标准》 (GB/T 14848–2017)规定的Ⅲ类水限值。 表 2 研究区地下水化学类型统计
Table 2. Statistics of groundwater hydrochemical types in study area
层位 水化学类型 样品数/组 比例/% 潜水 HCO3–Ca·Na 12 24.0 HCO3·Cl–Na 11 22.0 Cl·HCO3–Na 7 14.0 HCO3–Na 6 12.0 HCO3·SO4–Ca·Na 5 10.0 HCO3·SO4·Cl–Na·Ca 4 8.0 Cl·HCO3–Ca 2 4.0 Cl–Na 2 4.0 HCO3·Cl–Ca·Na 1 2.0 承压水 Cl–Na 4 28.6 HCO3·Cl–Na 3 21.4 Cl·HCO3–Na 3 21.4 Cl–Na·Mg 3 21.4 HCO3–Na 1 7.1 表 3 研究区潜水主成分分析
Table 3. PCA (Principal Component Analysis) of phreatic water in the study area
指标 PC1 PC2 PC3 TDS 0.961 0.166 0.022 K+ 0.752 0.387 0.015 Na+ 0.985 0.070 0.020 Ca2+ 0.300 0.630 −0.018 Mg2+ 0.938 0.297 −0.004 HCO3– 0.707 0.583 −0.100 Cl– 0.965 0.017 0.038 SO42– 0.144 0.038 −0.016 NH4+ 0.230 0.295 0.851 NO3– −0.192 −0.208 0.906 Mn 0.074 0.828 0.056 Br– 0.915 −0.092 0.026 特征值 7.42 1.571 1.51 方差贡献率/% 57.081 12.085 11.617 累积方差贡献率/% 57.081 69.165 80.782 表 4 饮水及皮肤接触健康风险评价结果
Table 4. Assessment results of health risks through drinking water intake and dermal contact
层位 化学
组分项目 HQoral HQdermal HI 成人 儿童 成人 儿童 成人 儿童 潜水 NH4+ 最大值 0.75 1.20 9.27×10−4 2.30×10−3 0.75 1.20 最小值 5.58×10−4 8.93×10−4 6.92×10−7 1.72×10−6 5.59×10−4 8.95×10−4 平均值 0.04 0.07 5.57×10−5 1.38×10−4 0.04 0.07 HI>1比例/% – – – – 0 2 Mn 最大值 1.03 1.64 1.36×10−3 2.15×10−3 1.03 1.65 最小值 1.19×10−3 1.90×10−3 1.58×10−6 2.49×10−6 1.19×10−3 1.91×10−3 平均值 0.20 0.32 2.62×10−4 4.13×10−4 0.20 0.32 HI>1比例/% – – – – 2 14 承压水 NH4+ 最大值 0.64 1.03 7.98×10−4 1.98×10−3 0.64 1.03 最小值 1.81×10−3 2.89×10−3 2.24×10−6 5.55×10−6 1.81×10−3 2.89×10−3 平均值 0.22 0.36 2.77×10−4 6.87×10−4 0.22 0.36 HI>1比例/% – – – – 0 7 Mn 最大值 4.46 7.14 5.93×10−4 9.34×10−4 4.47 7.15 最小值 1.25×10−2 2.00×10−2 1.66×10−5 2.62×10−5 1.25×10−2 2.00×10−2 平均值 1.55 2.48 2.06×10−4 3.24×10−4 1.55 2.48 HI>1比例/% – – – – 50 79 -
[1] Ahmed A, Clark I. 2016. Groundwater flow and geochemical evolution in the central Flinders Ranges, South Australia[J]. Science of the Total Environment, 572(1): 837−851.
[2] Benadela L, Bekkoussa B, Gaïdi L. 2022. Multivariate analysis and geochemical investigations of groundwater in a semi–arid region, case of Ghriss Basin superficial aquifer North–West Algeria[J]. Journal of Groundwater Science and Engineering, 10(3): 233−249.
[3] Bozau E, Haussler S, van Berk W. 2015. Hydrogeochemical modelling of corrosion effects and barite scaling in deep geothermal wells of the North German Basin using PHREEQC and PHAST[J]. Geothermics, 53: 540−547. doi: 10.1016/j.geothermics.2014.10.002
[4] Chebotarev I I. 1955. Metamorphism of natural waters in the crust of weathering–2[J]. Geochimica et Cosmochimica Acta, 8: 137−170. doi: 10.1016/0016-7037(55)90010-7
[5] Chen Zongyu, Wan Li, Nie Zhenlong, Shen Jianmei, Chen Jingsheng. 2006. Identification of groundwater recharge in the Heihe Basin using environmental isotopes[J]. Hydrogeology and Engineering Geology, (6): 9−14 (in Chinese with English abstract).
[6] Craig H. 1961. Isotopic variations in meteoric waters[J]. Science, 133: 1702−1703. doi: 10.1126/science.133.3465.1702
[7] Cui Jiaqi, Li Xianyue, Shi Haibin, Sun Yanan, An Haijun, Xing Jinping. 2020. Chemical evolution and formation mechanism of groundwater in Hetao Irrigation Area[J]. Environmental Science, 41(9): 4011−4020 (in Chinese with English abstract).
[8] Gan L, Huang G X, Pei L X, Gan Y J, Liu C Y, Yang M N, Han D Y, Song J M. 2022. Distributions, origins, and health–risk assessment of nitrate in groundwater in typical alluvial–pluvial fans, North China Plain[J]. Environmental Science and Pollution Research, 29(2): 17031−17048.
[9] Gibbs R J. 1970. Mechanisms controlling world water chemistry[J]. Science, 170(3962): 1088−1090. doi: 10.1126/science.170.3962.1088
[10] Guo Xiaojiao, Wang Huiwei, Shi Jiansheng, Wang Wei. 2022. Hydrochemical characteristics and evolution pattern of groundwater system in Baiyangdian wetland, North China Plain[J]. Acta Geologica Sinica, 96(2): 656−672 (in Chinese with English abstract).
[11] Hu Yunzhuang, Li Hong, Li Ying, Shi Peixin, Yang Jilong, Hu Ziyuan, Liu Hongwei. 2015. Hydrogeochemical recognition of seawater intrusion process at the typical profile in Laizhou Bay[J]. Geological Survey and Research, 38(1): 41−50 (in Chinese with English abstract).
[12] Huang G X, Sun J C, Zhang Y, Chen Z Y, Liu F. 2013. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China[J]. Science of The Total Environment, 463–464(5): 209–221.
[13] Ijumulana J., Ligate F, Bhattacharya P, Mtalo F, Zhang C. 2020. Spatial analysis and GIS mapping of regional hotspots and potential health risk of fluoride concentrations in groundwater of northern Tanzania[J]. Science of the Total Environment, 735: 139584. doi: 10.1016/j.scitotenv.2020.139584
[14] Koh D, Mayer B, Lee K, Ko K. 2010. Land–use controls on sources and fate of nitrate in shallow groundwater of an agricultural area revealed by multiple environmental tracers[J]. Journal of Contaminant Hydrology, 118(1/2): 62−78.
[15] Lei Ming, Zhang Shuijun, Zhu Zheng, Ku Hanpeng, Dong Xianzhe, Zhang Zejun. 2019. Characteristics of groundwater isotopes and renewability of groundwater in Jinhua Area[J]. Journal of China Hydrology, 39(6): 59−63 (in Chinese with English abstract).
[16] Li P Y., Wu J H, Qian H. 2016. Preliminary assessment of hydraulic connectivity between river water and shallow groundwater and estimation of their transfer rate during dry season in the Shidi River, China[J]. Environmental Earth Sciences, 75(2): 1−16.
[17] Li Zhuang, Su Jingwen, Dong Changchun, Ye Yonghong, Yang Yang. 2022. Hydrochemistry characteristics and evolution mechanisms of the groundwater in Dangtu area, Ma'anshan City, Anhui Province[J]. Geology in China, 49(5): 1509−1526 (in Chinese with English abstract).
[18] Liu Chunyan, Yu Kaining, Zhang Ying, Jing Jihong, Liu Jingtao. 2023. Characteristics and driving mechanisms of shallow groundwater chemistry in Xining City[J]. Environmental Science, 44(6): 3228−3236 (in Chinese with English abstract).
[19] Lu Xurong, Zhou Aiguo, Wang Maoting, Yang Lei, Lu Hua. 2010. Characteristic analysis of phreatic water equality evolution by Piper diagram in Huaihe river drainage area, Jiangsu Province[J]. Geotechnical Investigation and Surveying, 38(2): 42−47 (in Chinese with English abstract).
[20] Mao H R, Wang G C, Liao F, Shi Z M, Huang X J, Li B, Yan X. 2022. Geochemical evolution of groundwater under the influence of human activities: A case study in the southwest of Poyang Lake Basin[J]. Applied Geochemistry, 140: 105299. doi: 10.1016/j.apgeochem.2022.105299
[21] Meng Ruifang, Yang Huifeng, Bai Hua, Xu Buyun. 2023. Analysis of chemical characteristics and evolutionary patterns of groundwater in the Daqing River Plain Area of Haihe Basin[J]. Rock and Mineral Analysis, 42(2): 383−395 (in Chinese with English abstract).
[22] Pavlovskiy I, Selle B. 2015. Integrating hydrogeochemical, hydrogeological, and environmental tracer data to understand groundwater flow for a karstified aquifer systems[J]. Groundwater, 53: 156−165. doi: 10.1111/gwat.12262
[23] Pu Junbing, Yuan Daoxian, Jiang Yongjun, Gou Pengfei, Yin Jianjun. 2010. Hydrogeochemistry and environmental meaning of Chongqing subterranean karst streams in China[J]. Advances in Water Science, 21(5): 628−636 (in Chinese with English abstract).
[24] Reddy A G S, Kumar K, Niranjan. 2010. Identification of the hydrogeochemical processes in groundwater using major ion chemistry: A case study of Penna–Chitravathi river basins in Southern India[J]. Environmental Monitoring and Assessment, 170: 365−382. doi: 10.1007/s10661-009-1239-4
[25] Said I, Merz C, Salman A E, Schneider M, Winkler A. 2020. Identification of hydrochemical processes using multivariate statistics in a complex aquifer system of Sohag region, Egypt[J]. Environmental Earth Sciences, 79(8): 1−14.
[26] Sun X B, Guo C L, Zhang J, Sun J Q, Cui J, Liu M H. 2023. Spatial–temporal difference between nitrate in groundwater and nitrogen in soil based on geostatistical analysis[J]. Journal of Groundwater Science and Engineering, 11(1): 37−46. doi: 10.26599/JGSE.2023.9280004
[27] Ta M M, Zhou X, Guo J, Wang X Y, Xu Y Q. 2020. The evolution and sources of major ions in hot springs in the Triassic Carbonates of Chongqing, China[J]. Water, 12(4): 1194. doi: 10.3390/w12041194
[28] USEPA, 2008. User’s Guide: Human Health Risk Assessment[R]. Washington DC: United States Environmental Protection Agency.
[29] Wang H, Jiang X W, Wan L, Han G L, Guo H M. 2015. Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin[J]. Journal of Hydrology, 527: 433−441. doi: 10.1016/j.jhydrol.2015.04.063
[30] Wu Tong. 2019. Quaternary Strata and Paleoenvironmental Evolution in Coastal Plain of Wenzhou [D]. Chengdu: Chengdu University of Technology, 1–139 (in Chinese with English abstract).
[31] Xi Long, Chen Keheng, Huang Xiangqing, Xia Zhen, Tan Xiaoyu. 2021. Hydrogeochemistry and origin of groundwater in the south coast of Hainan[J]. Geological Bulletin of China, 40(2/3): 350−363 (in Chinese with English abstract).
[32] Xiao Y, Shao J L, Cui Y L, Zhang G, Zhang Q L. 2017. Groundwater circulation and hydrogeochemical evolution in Nomhon of Qaidam Basin, northwest China[J]. Journal of Earth System Science, 126(2): 1−16.
[33] Xiao Guoqiang, Yang Jilong, Hu Yunzhuang, Du Dong, Xu Qinmian, Qin Yafei, Fang Chen. 2014. Hydrogeochemical recognition of seawater intrusion processes in Yang River and Dai River coastal plain of Qinhuangdao City[J]. Safety and Environmental Engineering, 21(2): 32−39 (in Chinese with English abstract).
[34] Xiong G Y, Chen G Q, Wu J C, Wang Z Y, Yu H J, Fu T F, Liu W Q, Xu X Y, Hou G H, Yang Y, Zhu X B. 2022. Identifying the characteristics and potential risk of seawater intrusion for southern China by the SBM–DEA model[J]. Science of the Total Environment, 844: 157205. doi: 10.1016/j.scitotenv.2022.157205
[35] Xu Naizheng, Liu Hongying, Weifeng, Yang Hui, Geweiya. 2015. Study on the environmental isotope composition and their evolution in groundwater of Yoco port in Jiangsu Province, China[J]. Acta Scientiae Circumstantiae, 35(12): 3862−3871 (in Chinese with English abstract).
[36] Zhang Jingtao, Shi Zheming, Wang Guangcai, Jiang Jun, Yang Bingchao. 2021. Hydrochemical characteristic and evolution of groundwater in the Dachaidan area, Qaidam Basin[J]. Earth Science Frontiers, 28(4): 194−205 (in Chinese with English abstract).
[37] Zhang Xiaowen, He Jiangtao, Peng Cong, Zhang Changyan, Ni Zehua. 2017. Comparison of identification methods of main component hydrochemical anomalies in groundwater: A case study of Liujiang Basin[J]. Environmental Science, 38(8): 3225−3233 (in Chinese with English abstract).
[38] Zhang Y, Chen Z Y, Huang G X, Yang M N. 2023. Origins of groundwater nitrate in a typical alluvial–pluvial plain of North China Plain: new insights from groundwater age–dating and isotopic fingerprinting[J]. Environmental Pollution, 316: 120592. doi: 10.1016/j.envpol.2022.120592
[39] Zhang Y H, Dai Y S, Wang Y, Huang X, Yong X, Pei Q M. 2021 Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China[J]. Science of the Total Environment, 784: 147186
[40] Zhou B, Wang Huiwei, Zhang Qianqian. 2021. Assessment of the evolution of groundwater chemistry and its controlling factors in the Huangshui River Basin of Northwestern China, using hydrochemistry and multivariate statistical techniques[J]. International Journal of Environmental Research and Public Health, 18: 7551. doi: 10.3390/ijerph18147551
[41] Zhou Yangxiao, Li Wenpeng. 2011. Groundwater Monitoring Information System Model and Sustainable Development[M]. Beijing: Science Press, 45–48 (in Chinese).
[42] 陈宗宇, 万力, 聂振龙, 申建梅, 陈京生. 2006. 利用稳定同位素识别黑河流域地下水的补给来源[J]. 水文地质工程地质, (6): 9−14. doi: 10.3969/j.issn.1000-3665.2006.06.003
[43] 崔佳琪, 李仙岳, 史海滨, 孙亚楠, 安海军, 邢进平. 2020. 河套灌区地下水化学演变特征及形成机制[J]. 环境科学, 41(9): 4011−4020.
[44] 郭小娇, 王慧玮, 石建省, 王伟. 2022. 白洋淀湿地地下水系统水化学变化特征及演化模式[J]. 地质学报, 96(2): 656−672. doi: 10.3969/j.issn.0001-5717.2022.02.020
[45] 胡云壮, 李红, 李影, 施佩歆, 杨吉龙, 胡自远, 刘宏伟. 2015. 山东莱州湾南岸典型剖面海(咸)水入侵过程的水文地球化学识别[J]. 地质调查与研究, 38(1): 41−50. doi: 10.3969/j.issn.1672-4135.2015.01.006
[46] 雷明, 张水军, 珠正, 库汉鹏, 董贤哲, 章泽军. 2019. 金华地区地下水同位素特征及更新能力研究[J]. 水文, 39(6): 59−63.
[47] 李状, 苏晶文, 董长春, 叶永红, 杨洋. 2022. 安徽马鞍山市当涂地区地下水水化学特征及演化机制[J]. 中国地质, 49(5): 1509−1526. doi: 10.12029/gc20220510
[48] 刘春燕, 于开宁, 张英, 荆继红, 刘景涛. 2023. 西宁市浅层地下水化学特征及形成机制[J]. 环境科学, 44(6): 3228−3236.
[49] 陆徐荣, 周爱国, 王茂亭, 杨磊, 陆华. 2010. Piper图解淮河流域江苏地区浅层地下水水质演化特征[J] 工程勘查, 38(2): 42−47.
[50] 孟瑞芳, 杨会峰, 白华, 徐步云. 2023. 海河流域大清河平原区地下水化学特征及演化规律分析[J]. 岩矿测试, 42(2): 383−395.
[51] 蒲俊兵, 袁道先, 蒋勇军, 苟鹏飞, 殷建军. 2010. 重庆岩溶地下河水文地球化学特征及环境意义[J]. 水科学进展, 21(5): 628−636.
[52] 宋献方, 刘相超, 夏军, 于静洁, 唐常源. 2007. 基于环境同位素技术的怀沙河流域地表水和地下水转化关系研究[J]. 中国科学(D辑: 地球科学), (1): 102−110.
[53] 王可欣, 楼俊伟, 俞涵婷. 2020. 近50a浙江降水分布的时空特征[J]. 浙江气象, 42(1): 11−16.
[54] 吴同. 2019. 温州沿海平原第四纪地层及古环境演变[D]. 成都: 成都理工大学, 1−139.
[55] 习龙, 陈科衡, 黄向青, 甘华阳, 夏真, 谭晓煜. 2021. 海南南部沿海地下水水文地球化学及成因[J]. 地质通报, 40(2/3): 350−363.
[56] 肖国强, 杨吉龙, 胡云壮, 杜东, 胥勤勉, 秦雅飞, 方成. 2014. 秦皇岛洋−戴河滨海平原海水入侵过程水文化学识别[J]. 安全与环境工程, 21(2): 32−39. doi: 10.3969/j.issn.1671-1556.2014.02.008
[57] 许乃政, 刘红樱, 魏峰, 杨辉, 葛伟亚. 2015. 江苏洋口港地区地下水的环境同位素组成及其形成演化研究[J]. 环境科学学报, 35(12): 3862−3871.
[58] 张景涛, 史浙明, 王广才, 姜军, 杨炳超. 2021. 柴达木盆地大柴旦地区地下水水化学特征及演化规律[J]. 地学前缘, 28(4): 194−205.
[59] 张小文, 何江涛, 彭聪, 张昌延, 倪泽华. 2017. 地下水主要组分水化学异常识别方法对比: 以柳江盆地为例[J]. 环境科学, 38(8): 3225−3233.
[60] 周仰效, 李文鹏. 2011. 地下水监测信息系统模型及可持续开发[M]. 北京: 科学出版社, 45−48.
-




 下载:
下载: