Effect of Salt Medium on Preparation of α-hemihydrate from Phosphogypsum Using Salt Solution Method
-
摘要:
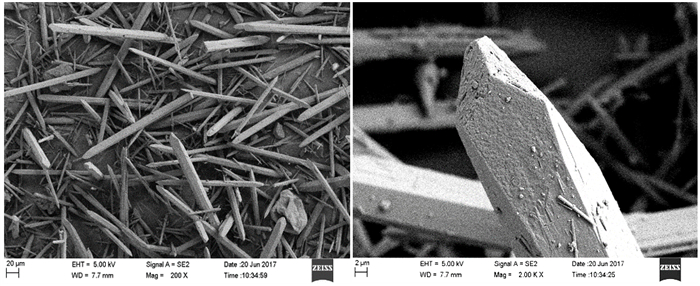
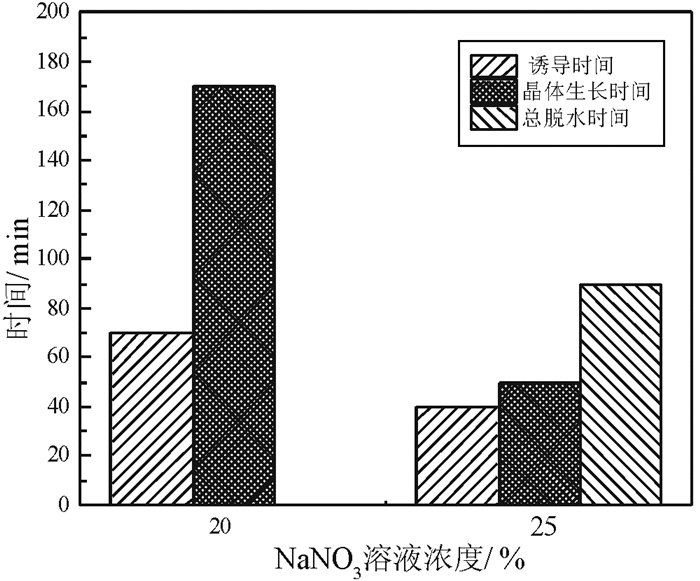
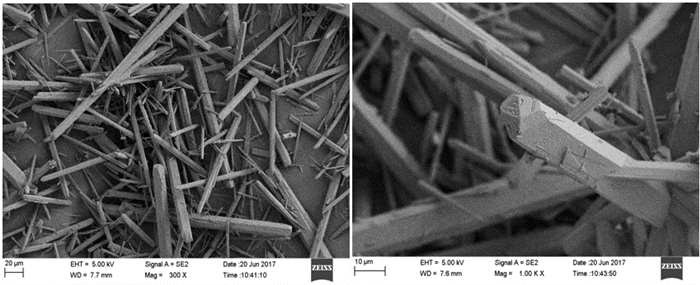
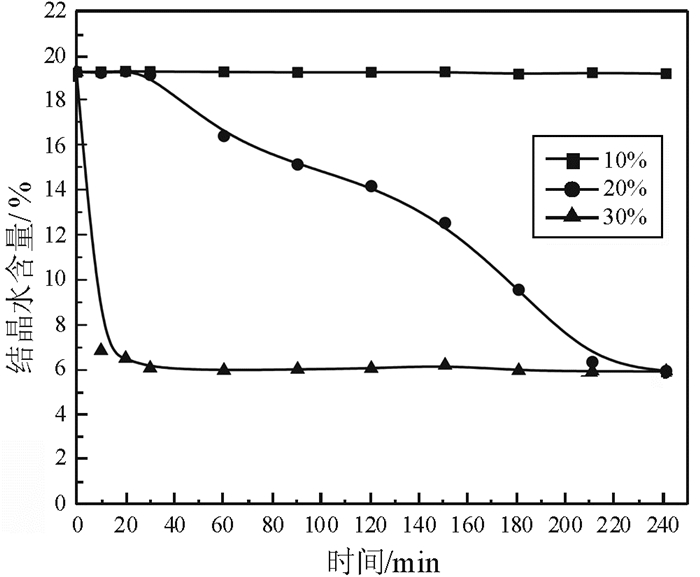
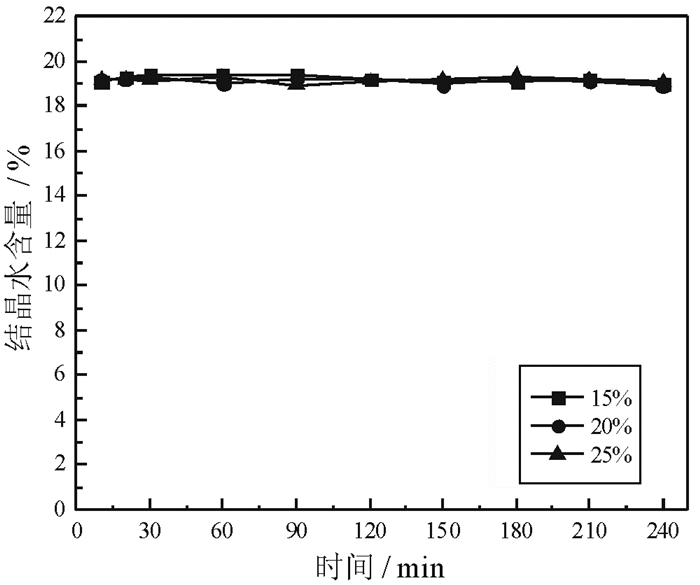
磷石膏是湿法磷酸生产过程中用硫酸分解磷矿石排放的固体废弃物,采用常压盐溶液法制备α半水石膏是磷石膏资源化的新途径,其中盐介质的选择是常压盐溶液法的关键。研究了NaCl、NaNO3、MgCl2、CaCl2和Ca(NO3)2五种盐介质对磷石膏制备α半水石膏转化速率、物相组成和形貌的影响。研究结果表明:随着NaCl、NaNO3和MgCl2浓度的增加,磷石膏转化为α半水石膏的速率加快,结晶诱导时间和晶体生长时间缩短;反应产物中α半水石膏的含量在95%以上,显微形貌均为六方长柱状,但端面形貌不同。在NaCl和NaNO3溶液中,α半水石膏存在多个锥面且晶面完整,长径比约为14:1;在MgCl2溶液中端面呈空心层状包裹,长径比为11:1,粒度均匀,但晶体缺陷较大。NaCl与NaNO3、MgCl2相比,具有用量低、磷石膏转化速率快的优点,可作为常压盐溶液制备α半水石膏的盐介质;由于同离子效应的影响,磷石膏在CaCl2和Ca(NO3)2溶液中未发生转变,不宜作为盐介质。
Abstract:Phosphogypsum (PG) is an industrial solid waste from wet preparation of phosphoric acid using sulfuric acid to decompose phosphate ore. Preparation of α-hemihydrate gypsum(α-HH) using salt solution method is a new utilization way to recycle PG, in which the selection of salt media plays an important role. Therefore, the conversion rate, phase composition and morphology of α-HH in NaCl, NaNO3, MgCl2, CaCl2 and Ca(NO3)2 solution were investigated in this paper. The results show that the conversion rate of α-HH increases with increasing the concentration of NaCl, NaNO3 and MgCl2, which shortens the crystallization induction time and crystal growth time. The content of α-HH in product is above 95%, of which the morphology is long hexagonal prism with different end face. In NaCl and NaNO3 solutions, the α-HH present complete and polyhedral crystal face with a length-diameter ratio (L/D) of 14:1. While in MgCl2 solution, the end face is packed hollow pipe with the L/D of 11:1, which is uniformly distributed with large crystal defect. Compared with NaNO3 and MgCl2, NaCl has the advantages of low dosage and high conversion rate, which can be used as the salt medium for the preparation of α-HH. Due to the common-ion effect, PG can't convert to α-HH in CaCl2 and Ca(NO3)2 solutions, which are not suitable as salt media.
-
Key words:
- phosphogypsum /
- salt solution method /
- α-hemihydrate gypsum /
- salt medium
-

-
表 1 磷石膏化学组成
Table 1. Chemical component of phosphogypsum
化学组成 CaO Fe2O3 Al2O3 SiO2 SO3 P2O5 MgO F 结晶水 其他 含量/% 34.07 0.20 0.13 5.29 40.24 0.75 0.01 0.14 19.11 0.06 表 2 不同NaCl浓度下反应产物结构相的半定量检索结果
Table 2. Semi quantitative results of the phases of reaction products in different NaCl concentrations
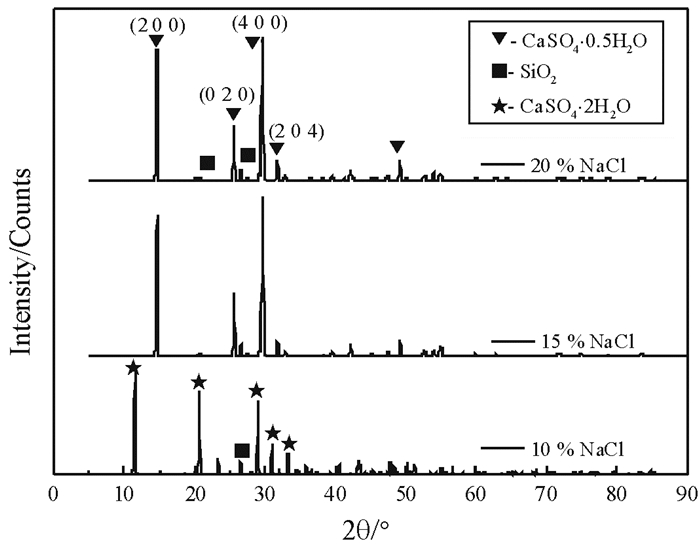
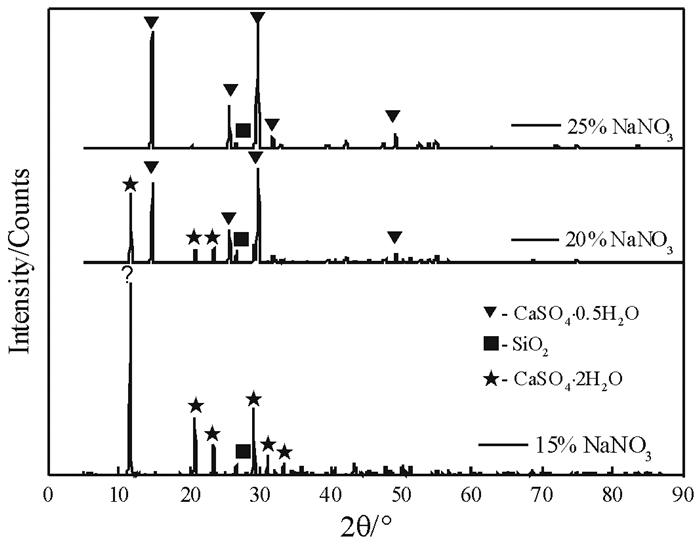
NaCl浓度/% 结构相 代码 半定量/% 10 CaSO4·2H2O 00-033-0311 91 SiO2 01-079-1910 9 15 CaSO4·0.5H2O 96-901-2210 96 SiO2 01-079-1910 4 20 CaSO4·0.5H2O 96-901-2210 95 SiO2 01-079-1910 5 表 3 不同NaNO3浓度下反应产物结构相的半定量检索结果
Table 3. Semi quantitative results of the phases of reaction products in different NaNO3 concentrations
NaNO3浓度/% 结构相 代码 半定量/% 15 CaSO4·2H2O 00-033-0311 92 SiO2 01-079-1910 8 20 CaSO4·0.5H2O 96-901-2210 76 CaSO4·2H2O 00-033-0311 19 SiO2 01-079-1910 5 25 CaSO4·0.5H2O 96-901-2210 98 SiO2 01-079-1910 2 表 4 不同MgCl2浓度下反应产物结构相的半定量检索结果
Table 4. Semi quantitative results of the phases of reaction products in different MgCl2 concentrations
MgCl2浓度/% 结构相 代码 半定量/% 10 CaSO4·2H2O 00-033-0311 95 SiO2 01-079-1910 5 20 CaSO4·0.5H2O 96-901-2210 97 SiO2 01-079-1910 3 30 CaSO4·0.5H2O 96-901-2210 96 SiO2 01-079-1910 4 -
[1] Tayibi H, Choura M, López F A, et al. Environmental impact and management of phosphogypsum[J]. Journal of Environmental Management, 2009, 90(8):2377-2386. doi: 10.1016/j.jenvman.2009.03.007
[2] 侯婷婷, 郑传阳, 江晓敏.固体废弃物磷石膏的净化处理及石膏晶须的研究[J].安徽化工, 2015, 41(5):46-49. http://www.wenkuxiazai.com/doc/733496e5453610661ed9f4e7.html
[3] Shen Y, Qian J, Chai J, et al. Calcium sulphoaluminate cements made with phosphogypsum: production issues and material properties[J]. Cement and Concrete Composites, 2014, 48(4): 67-74. https://www.sciencedirect.com/science/article/pii/S0958946514000201
[4] 桂明生, 王鹏飞, 董俊.脱色磷石膏制备硫酸钙晶须的研究[J].贵州大学学报(自然科学版), 2014, 31(3):59-61. http://www.cqvip.com/QK/95366X/201603/668252267.html
[5] Guan B, Yang L, Wu Z, et al. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions [J]. Fuel, 2009, 88(7):1286-1293. doi: 10.1016/j.fuel.2009.01.004
[6] 茹晓红. 磷石膏基胶凝材料的制备理论及应用技术研究[D]. 武汉: 武汉理工大学, 2013.
http://cdmd.cnki.com.cn/Article/CDMD-10497-1013297455.htm [7] 刘红霞. 常压盐溶液法α-半水脱硫石膏的制备及晶形调控研究[D]. 重庆: 重庆大学, 2010.
http://cdmd.cnki.com.cn/Article/CDMD-10611-2010215874.htm [8] Jiang G, Wang H, Chen Q, et al. Preparation of alpha-calcium sulfate hemihydrate from FGD gypsum in chloride-free Ca(NO3)2 solution under mild conditions[J]. Fuel, 2016, 174: 235-241. doi: 10.1016/j.fuel.2016.01.073
[9] Guan B, Kong B, Fu H, et al. Pilot scale preparation of alpha-calcium sulfate hemihydrate from FGD gypsum in Ca-K-Mg aqueous solution under atmospheric pressure[J]. Fuel, 2012, 98: 48-54. doi: 10.1016/j.fuel.2012.03.032
-




 下载:
下载: