Preparation of Expanded Microcrystalline Graphite and Its Adsorption Behavior of Pb2+
-
摘要:
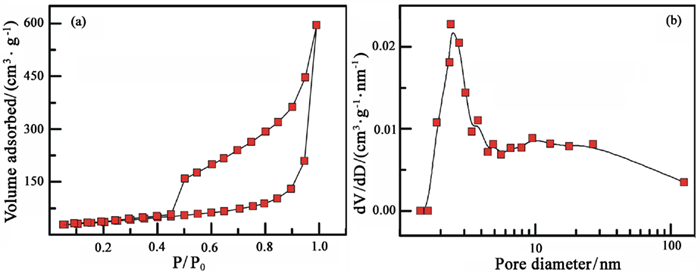
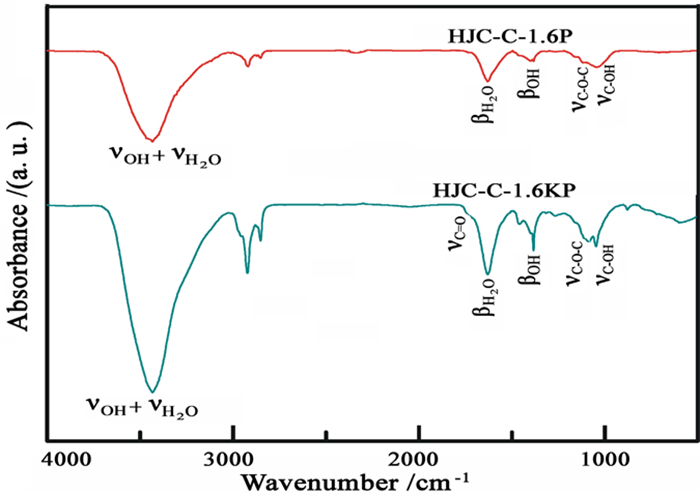
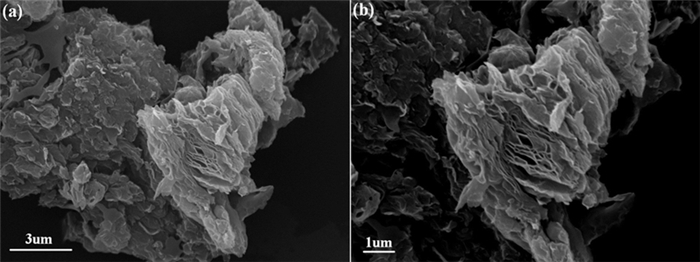
为进一步拓展微晶石墨的应用领域,将其氧化-膨胀获得膨胀微晶石墨,并用于含铅废水处理。通过静态吸附试验考察初始Pb2+浓度、反应时间、pH和温度等因素对膨胀微晶石墨吸附性能的影响。结果表明,微晶石墨经氧化膨胀制备的膨胀微晶石墨颗粒呈"蠕虫"状,含有丰富的网络孔隙结构,孔径集中在2~5 nm。膨胀微晶石墨对Pb2+的吸附行为受初始浓度、时间、pH和温度的影响,吸附量与初始浓度、时间和pH呈正相关,与温度呈负相关。Langmuir等温吸附模型和准一级动力学模型较好地拟合了吸附过程。热力学分析表明,膨胀微晶石墨对Pb2+的吸附为自发进行的放热吸附过程,以物理吸附为主。
Abstract:To further extend the application of microcrystalline graphite, the expanded microcrystalline graphite was prepared by oxidation and expansion, and then was used in the treatment of lead bearing wastewater. The effects of initial Pb2+ concentration, reaction time, pH and temperature on the adsorption properties of expanded microcrystalline graphite were investigated by static adsorption experiments. The results showed that the expanded microcrystalline graphite was worm-shaped and composed of abundant network pores. The pore size was concentrated at 2~5 nm. The adsorption behavior of expanded microcrystalline graphite on Pb2+ was affected by initial concentration, time, pH and temperature. The adsorption capacity of expanded microcrystalline graphite was positively correlated with initial concentration, time and pH, and negatively correlated with temperature. The adsorption process fits well with the Langmuir isotherm model and pseudo-first-order kinetic model. Thermodynamic study revealed that adsorption of Pb2+ on the expanded microcrystalline graphite was primarily due to a spontaneous exothermic reaction, and the process was mainly controlled by physical adsorption.
-
Key words:
- microcrystalline graphite /
- oxidation /
- expansion /
- reduction /
- adsorption
-

-
表 1 微晶石墨样品的化学成分分析
Table 1. Chemical composition of microcrystalline graphite sample
成分 C Al2O3 CaO K2O TiO2 MnO SiO2 Fe2O3 MgO Na2O P2O5 LOI 含量/% 81.54 3.95 0.41 0.38 0.21 0.01 7.85 1.06 0.14 0.14 0.05 4.56 注:LOI为烧失量。 表 2 膨胀微晶石墨吸附Pb2+的等温模型参数
Table 2. Isothermal model parameters of Pb2+ adsorption on the expanded microcrystalline graphite
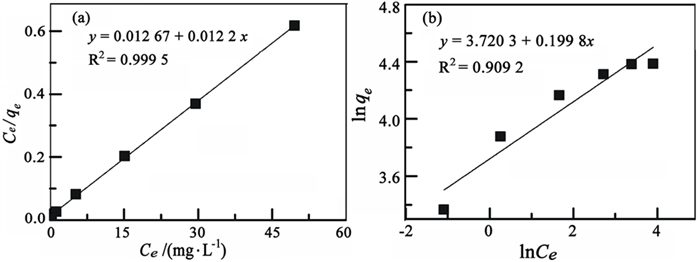
T/K 样品编号 Langmuir Freundlich qm kl R2 kf 1/n R2 298.15 HJC-C-1.6P 81.96 0.96 0.999 5 41.28 0.199 0.909 2 表 3 膨胀微晶石墨吸附Pb2+的动力学参数
Table 3. Kinetic parameters of Pb2+ adsorption on the expanded microcrystalline graphite
样品编号 准一级动力学 准二级动力学 qe k1 R2 qe k2 R2 HJC-C-1.6P 78.36 6.698 0.999 1 80.19 7.58×10-4 0.995 5 表 4 膨胀微晶石墨吸附Pb2+的热力学参数
Table 4. Thermodynamic parameters of Pb2+ adsorption on the expanded microcrystalline graphite
样品编号 ΔH°/
(kJ·mol-1)ΔS°/
(J·mol-1·K-1)ΔG°/(KJ·mol-1) R2 293 298 303 308 313 HJC-C-1.6P -12.48 -28.16 -4.16 -4.10 -4.02 -3.81 -3.60 0.961 3 -
[1] Kadirvelu K, Thamaraiselvi K, Namasivayam C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste[J]. Bioresource technology, 2001, 76(1):63-65. doi: 10.1016/S0960-8524(00)00072-9
[2] Li Y H, Wang S, Wei J, et al. Lead adsorption on carbon nanotubes[J]. Chemical physics letters, 2002, 357(3-4):263-266. doi: 10.1016/S0009-2614(02)00502-X
[3] Madadrang C J, Kim H Y, Gao G, et al. Adsorption behavior of EDTA-graphene oxide for Pb (Ⅱ) removal[J]. ACS applied materials & interfaces, 2012, 4(3):1186-1193. http://europepmc.org/abstract/MED/22304446
[4] Rao M M, Ramana D K, Seshaiah K, et al. Removal of some metal ions by activated carbon prepared from phaseolus aureus hulls[J]. Journal of hazardous materials, 2009, 166(2-3):1006-1013. doi: 10.1016/j.jhazmat.2008.12.002
[5] Solanki P, Gupta V, Kulshrestha R. Synthesis of zeolite from fly ash and removal of heavy metal ions from newly synthesized zeolite[J]. Journal of chemistry, 2010, 7(4):1200-1205. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_09f45f55557cd765e40808cdf858454f
[6] Cai G B, Zhao G X, Wang X K, et al. Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water[J]. The journal of physical chemistry C, 2010, 114(30):12948-12954. doi: 10.1021/jp103464p
[7] Fu F, Zeng H, Cai Q, et al. Effective removal of coordinated copper from wastewater using a new dithiocarbamate-type supramolecular heavy metal precipitant[J]. Chemosphere, 2007, 69(11):1783-1789. doi: 10.1016/j.chemosphere.2007.05.063
[8] Yang Z, Xia Y, Mokaya R. Enhanced hydrogen storage capacity of high surface area zeolite-like carbon materials[J]. Journal of the American chemical society, 2007, 129(6):1673-1679. doi: 10.1021/ja067149g
[9] Chae H K, Siberio-perez D Y, Kim J, et al. A route to high surface area, porosity and inclusion of large molecules in crystals[J]. Nature, 2004, 427(6974):523-527. doi: 10.1038/nature02311
[10] Wang Y, Li L, Luo C, et al. Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb2+[J]. International journal of biological macromolecules, 2016, 86:505-511. doi: 10.1016/j.ijbiomac.2016.01.035
[11] Yao S, Zhang J, Shen D, et al. Removal of Pb(Ⅱ) from water by the activated carbon modified by nitric acid under microwave heating[J]. Journal of colloid & interface science, 2016, 463:118-127. http://cn.bing.com/academic/profile?id=6396c9d271e5052a8ccc47eac1b4bd3a&encoded=0&v=paper_preview&mkt=zh-cn
[12] Li B Q, Yuan W H, Li L. Adsorption of Pb2+ and Cd2+ on graphene nanosheets prepared using thermal exfoliation[J]. Acta physico-chimica sinica, 2016, 32(4):997-1004. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb201604035
[13] 赵颖华.膨胀石墨的制备及其对金属离子去除性能的研究[D].上海: 东华大学, 2012: 24-40.
http://cdmd.cnki.com.cn/Article/CDMD-10255-1012311637.htm [14] 段佳琪, 孙红娟, 彭同江.超声-混酸法提纯微晶石墨[J].非金属矿, 2017, 40(1):58-61. doi: 10.3969/j.issn.1000-8098.2017.01.018
[15] McAllister M J, Li J L, Adamson D H, et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite[J]. Chemistry of materials, 2007, 19(18):4396-4404. doi: 10.1021/cm0630800
[16] Deng S, Ting Y P. Characterization of PEI-modified biomass and biosorption of Cu(Ⅱ), Pb(Ⅱ) and Ni(Ⅱ)[J]. Water research, 2005, 39(10):2167-2177. doi: 10.1016/j.watres.2005.03.033
[17] Huang X, Pan M. The highly efficient adsorption of Pb(Ⅱ) on graphene oxides:a process combined by batch experiments and modeling techniques[J]. Journal of molecular liquids, 2016, 215:410-416. doi: 10.1016/j.molliq.2015.12.061
[18] Li Y, Liu F, Xia B, et al. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites[J]. Journal of hazardous materials, 2010, 177(1):876-880. http://cn.bing.com/academic/profile?id=4c22b3161ab32f4fd07972a2f40a49cc&encoded=0&v=paper_preview&mkt=zh-cn
[19] Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American chemical society, 1918, 40(9):1361-1403. doi: 10.1021/ja02242a004
[20] Freundlich H M F. Over the adsorption in solution[J]. Journal of chemical physics, 1906, 57:385-470. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_1cd3104bdb3bf53e388ca9b91c94d854
[21] Yin Z, Xiong J, Chen M, et al. Recovery of uranium(Ⅵ) from aqueous solution by amidoxime functionalized wool fibers[J]. Journal of radioanalytical & nuclear chemistry, 2016, 307(2):1471-1479. https://www.researchgate.net/publication/314241469_Removal_of_hexavalent_chromium_ions_from_aqueous_solution_by_amidoxime_functionalized_wool_fibers
[22] Ho Y S. Review of second-order models for adsorption systems[J]. Journal of hazardous materials, 2006, 136(3):681-689. doi: 10.1016/j.jhazmat.2005.12.043
[23] Wang D, Liu L, Jiang X, et al. Adsorbent for p-phenylenediamine adsorption and removal based on graphene oxide functionalized with magnetic cyclodextrin[J]. Applied surface science, 2015, 329(1):197-205. http://cn.bing.com/academic/profile?id=f1e82009ef941b959d1d0458b51a4156&encoded=0&v=paper_preview&mkt=zh-cn
[24] Fernandes A N, Almeida C A P, Debacher N A, et al. Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat[J]. Journal of molecular structure, 2010, 982(1):62-65. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0217350182
-



 下载:
下载: