Experimental Study on the Preparation of High Purity Graphite by Alkali-acid Method
-
摘要:
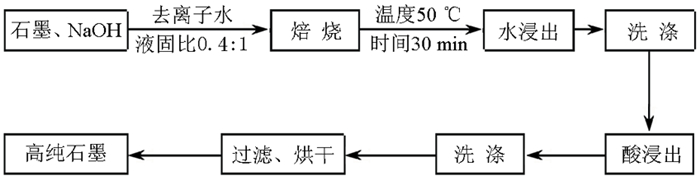
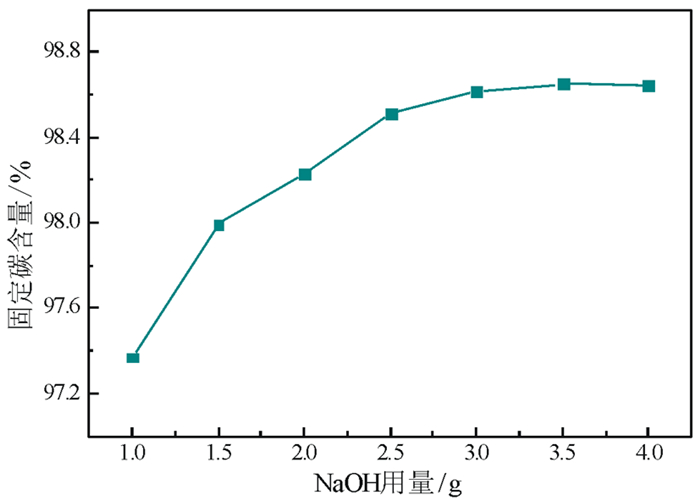
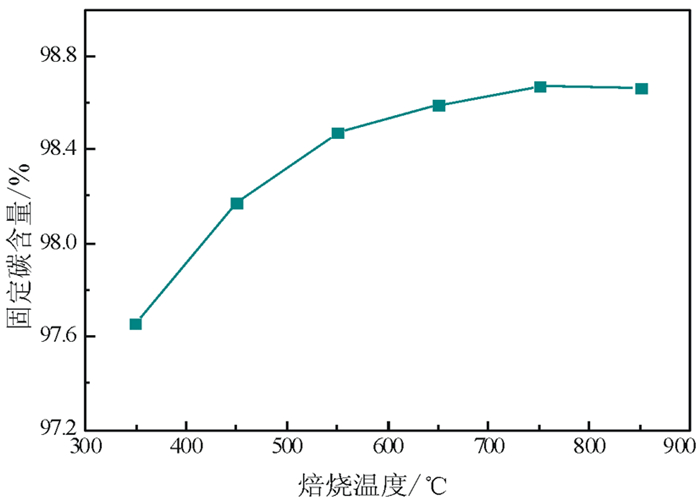
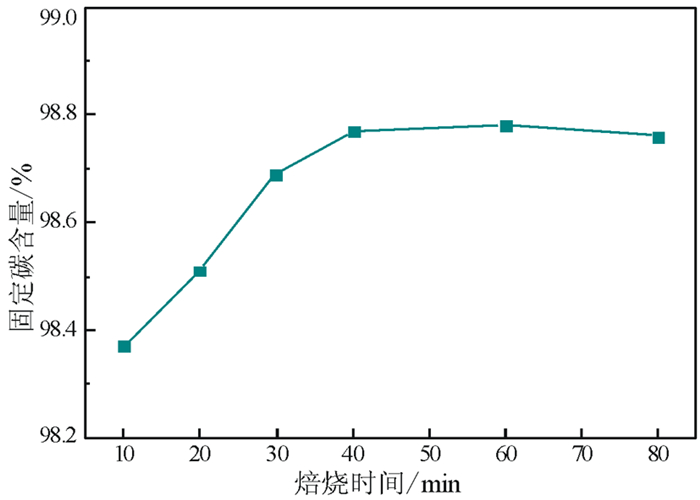
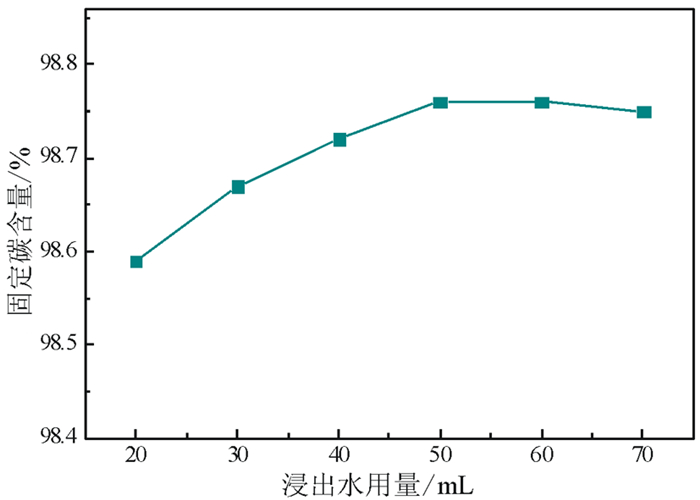
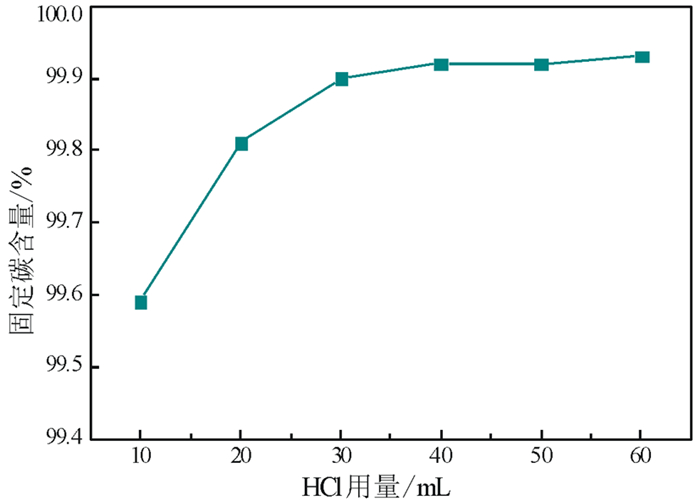
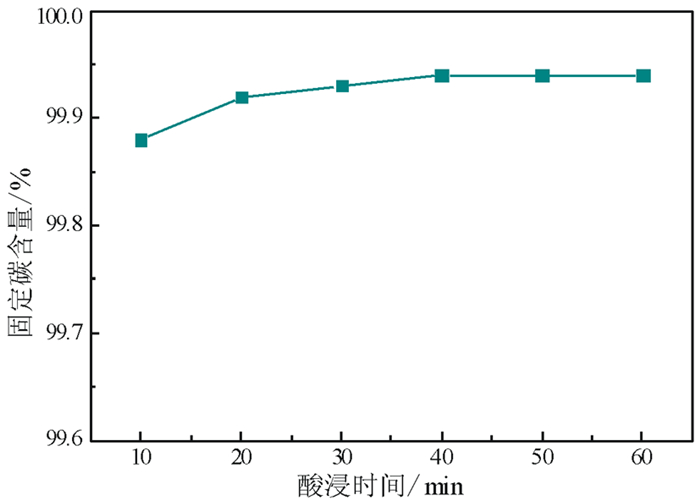
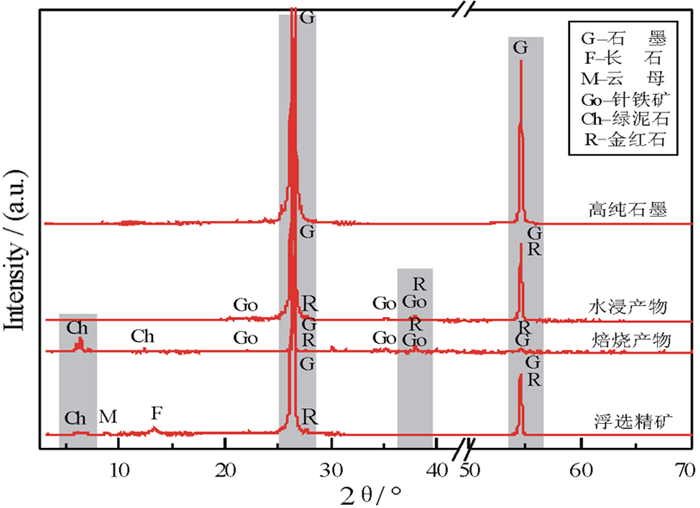
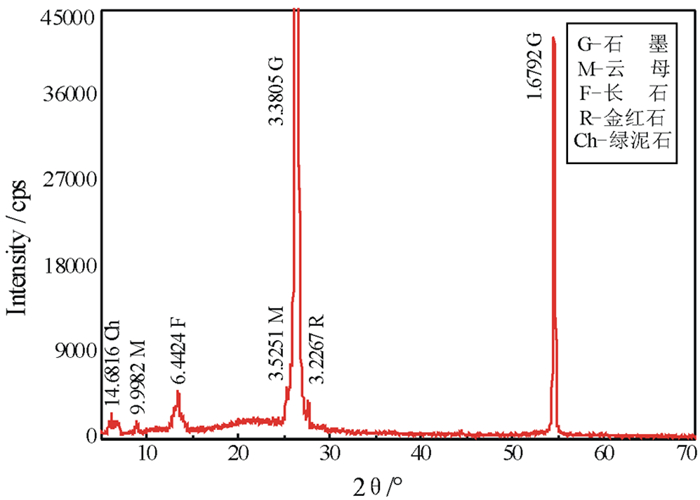
以黑龙江某地细鳞片石墨浮选精矿为原料进行碱酸法提纯试验,探讨了碱酸法提纯的最佳工艺条件。研究表明:在NaOH用量3.0 g(碱固比0.6:1)、焙烧温度750℃、焙烧时间40 min、浸出水用量50 mL、酸浸HCl浓度1.0 mol/L、用量40 mL、酸浸时间40 min的条件下,通过碱熔焙烧-水浸出-酸浸的工艺可将石墨固定碳含量由95.89%提升至99.94%。随着反应的进行以及物相的变化,杂质最终演变成可溶性物质,以洗涤的方式被去除;水浸出过程中保持弱碱性环境,有利于硅酸钠的溶解。
Abstract:Experiments were carried out on a fine flaky graphite flotation concentrate in Heilongjiang Provence by alkali-acid purification method. The optimum technological parameters were investigated. The results showed that under the conditions of NaOH dosage 3.0 g (ratio of alkali to solid 0.6:1), roasting temperature 750℃, roasting time 40 min, leaching water dosage 50 mL, acid leaching HCl concentration 1.0 mol/L, dosage 40 mL and acid leaching time 40 min, the fixed carbon content in graphite was increased from 95.89% to 99.94% by the process of alkali fusion roasting-water leaching-acid leaching. As the reaction proceeds and the phase changes, the impurities are eventually transformed into soluble matters and are washed with pure water. Maintaining a weak alkaline environment during water leaching is beneficial to the dissolution of sodium silicate.
-
Key words:
- graphite /
- purification /
- alkali-acid method /
- sodium silicate /
- mechanism
-

-
表 1 浮选精矿化学成分分析
Table 1. Chemical composition analysis of flotation concentrate
成分 SiO2 TiO2 Al2O3 Fe2O3 P2O5 MgO CaO V2O5 K2O SO3 ZnO Cl ZrO2 BaO 烧失量 固定碳 含量 0.85 0.39 0.33 0.24 0.18 0.098 0.060 0.049 0.039 0.036 0.016 0.009 0.001 0.001 97.70 95.89 注:根据GB/T 3521—2008石墨化学分析方法实验室自测固定碳含量。 -
[1] Wang H, Feng Q, Tang X, et al. Preparation of high-purity graphite from a fine microcrystalline graphite concentrate:effect of alkali roasting pre-treatment and acid leaching process[J]. Separation science & technology, 2016, 51(14):2465-2472. http://cn.bing.com/academic/profile?id=01a694ad1653dda003c78f1a9964b027&encoded=0&v=paper_preview&mkt=zh-cn
[2] 杜轶伦, 张福良.我国石墨资源开发利用现状及供需分析[J].矿产保护与利用, 2017(6):109-116. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=72006a00-89bc-47a4-abb3-9a3ffdf82a2e
[3] 王星, 胡立嵩, 夏林, 等.石墨资源概况与提纯方法研究[J].化工时刊, 2015, 29(2):19-22. doi: 10.3969/j.issn.1002-154X.2015.02.006
[4] 滕飞.高压碱浸-常压酸浸法提纯鳞片石墨的研究[D].昆明: 昆明理工大学, 2015.
[5] 罗立群, 谭旭升, 田金星.石墨提纯工艺研究进展[J].化工进展, 2014, 33(8):2110-2116. http://d.old.wanfangdata.com.cn/Periodical/hgjz201408031
[6] 张鸿波, 李悦, 张忠新.焙烧活化隐晶质石墨提纯试验研究[J].矿产综合利用, 2014(1):61-64. doi: 10.3969/j.issn.1000-6532.2014.01.015
[7] 张琳, 方建军, 赵敏捷, 等.隐晶质石墨提纯研究进展[J].化工进展, 2017, 36(1):261-267. http://d.old.wanfangdata.com.cn/Periodical/hgjz201701033
[8] 魏丽丹, 张文斌.石墨提纯方法现状及发展趋势[J].黑龙江生态工程职业学院学报, 2013, 26(6):26, 96. http://d.old.wanfangdata.com.cn/Periodical/hljstgczyxyxb201306012
[9] 姜芳, 涂文懋.碱酸法提纯某微晶石墨[J].金属矿山, 2014, 32(9):82-84. http://d.old.wanfangdata.com.cn/Periodical/jsks201409019
[10] 荆正强, 胡瑞彪.莫桑比克某球形石墨提纯试验[J].现代矿业, 2015, 31(3):230-232. doi: 10.3969/j.issn.1674-6082.2015.03.082
[11] 谭旭升.碱酸法提纯石墨及除硅动力学研究[D].武汉: 武汉理工大学, 2015.
http://cdmd.cnki.com.cn/Article/CDMD-10497-1015811638.htm [12] 李小波, 涂文懋, 胡鸿雁.隐晶质石墨提纯试验研究[J].炭素技术, 2013, 32(5):23-26. http://d.old.wanfangdata.com.cn/Periodical/kczhly201401015
[13] 袁韵茹, 张凌燕, 邱杨率, 等.莫桑比克大鳞片石墨化学提纯试验研究[J].硅酸盐通报, 2017, 36(8):2600-2606. http://d.old.wanfangdata.com.cn/Periodical/gsytb201708015
[14] Ge P, Wang H J, Zhao J, et al. Preparation of high purity graphite by an alkaline roasting-leaching method[J]. New carbon materials, 2010, 25(1):22-28. http://cn.bing.com/academic/profile?id=0d2a1aabdae63b05e2046f14c29e51f5&encoded=0&v=paper_preview&mkt=zh-cn
[15] 饶强, 戴朝成, 张怀胜, 等.绿泥石在三大岩中的赋存状态和成因[J].四川地质学报, 2016, 36(4):561-566. doi: 10.3969/j.issn.1006-0995.2016.04.007
[16] 刘海波.热处理铝代针铁矿的结构演化及其表面反应性[D].合肥: 合肥工业大学, 2013.
http://cdmd.cnki.com.cn/Article/CDMD-10359-1014120888.htm [17] Wu Z S, Ren W, Gao L, et al. Synthesis of graphene sheets with high electrical conductivity and good thermal stability by hydrogen arc discharge exfoliation[J]. ACS nano, 2009, 3(2):411-417. doi: 10.1021/nn900020u
[18] 杨晓峰.白钨矿与含钙脉石分离抑制剂的遴选及作用机理研究[D].昆明: 昆明理工大学, 2015.
http://cdmd.cnki.com.cn/Article/CDMD-10674-1016036982.htm -



 下载:
下载: