Study on the Influence of the Outlet and Inlet Structure of Ammonia Leaching Complexation Tank on the Complexation Concentration
-
摘要:
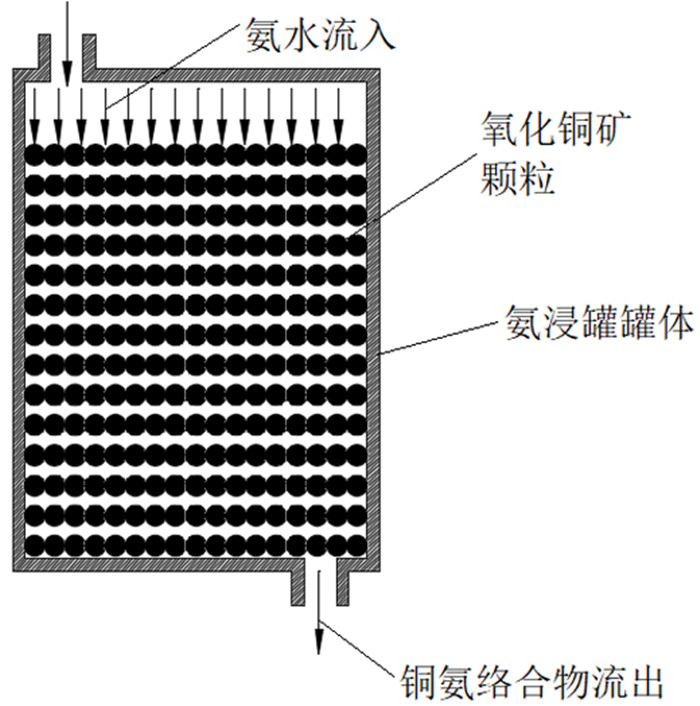
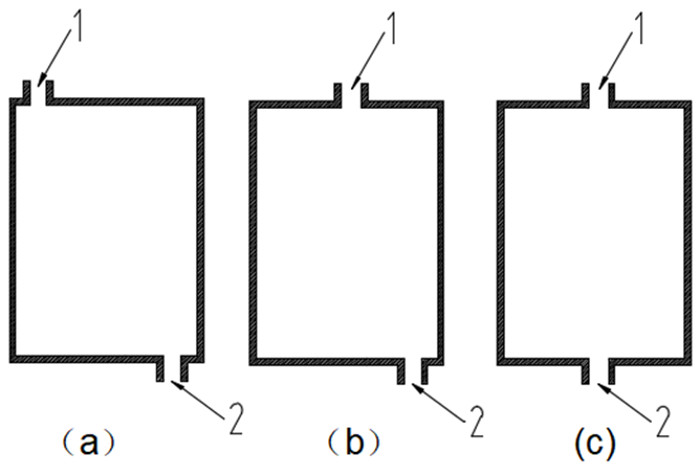
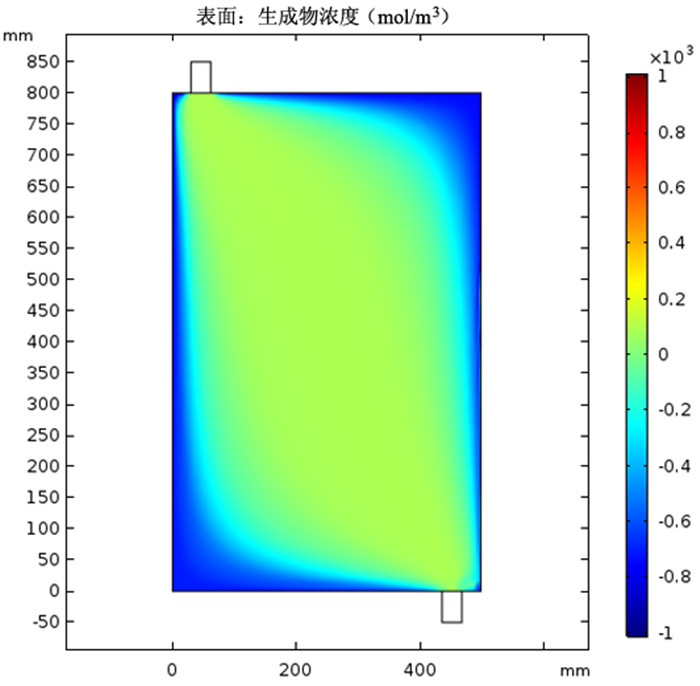
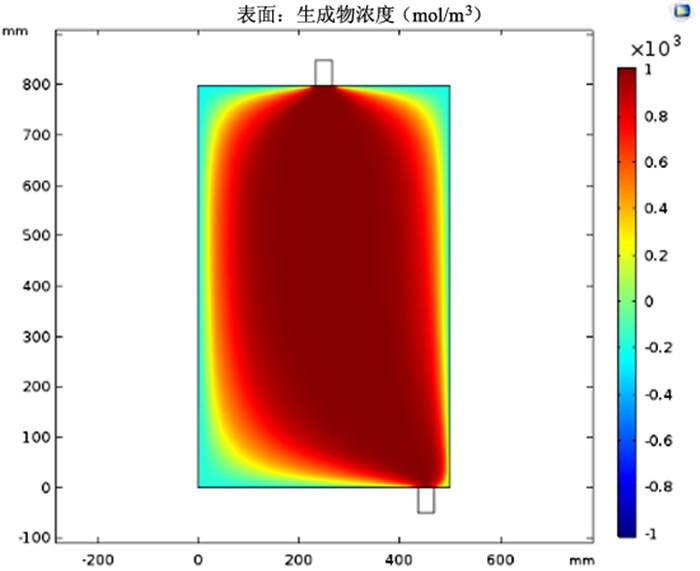
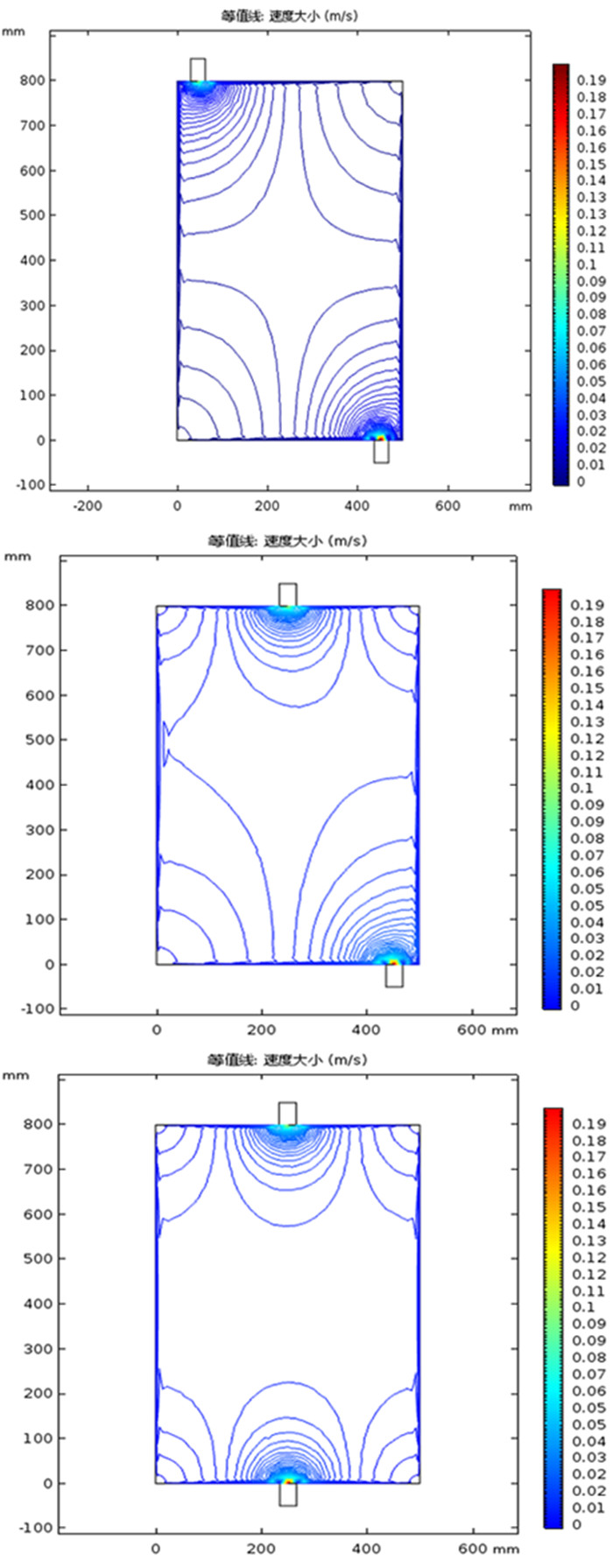
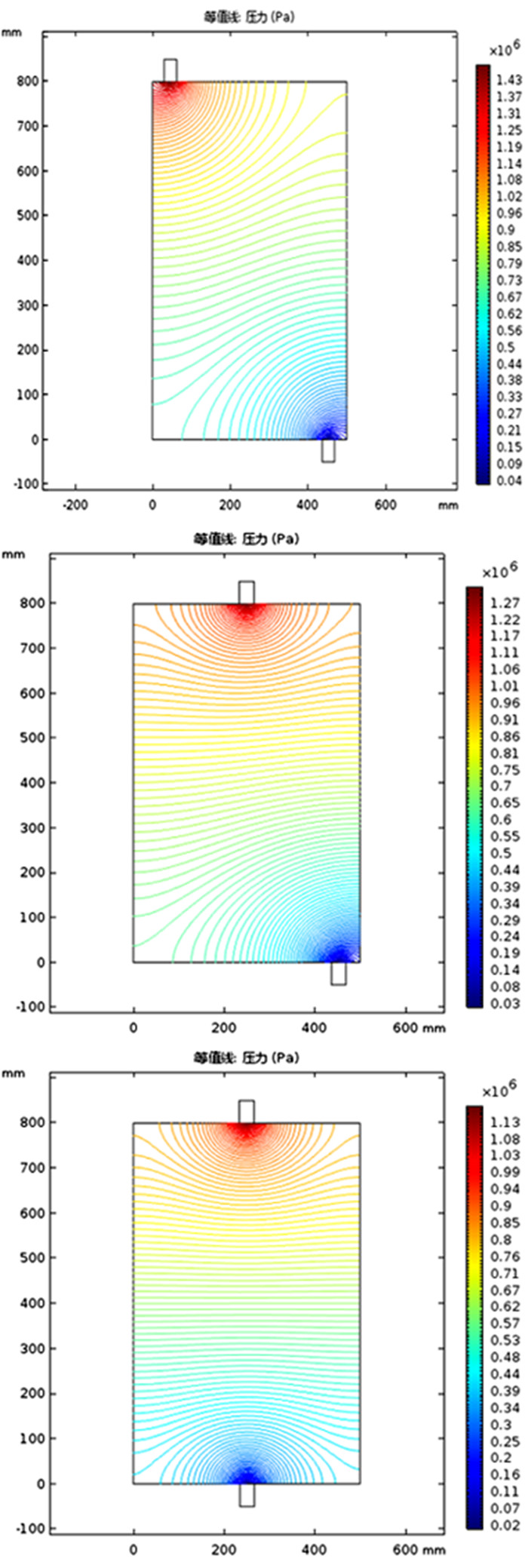
利用多孔介质多场耦合、化学反应传质等相关理论,采用Comsol Multiphysics仿真软件进行数值模拟,研究了氨浸过程中氨水在氧化铜矿石颗粒之间的流速、压强和生成物浓度,文章探讨的模型分别从罐体中部的左、中、右三个部位设置入口,罐体下部的右侧和中测设置出口,考察了氨浸罐不同出口和入口位置对氨浸过程中铜氨络合物浓度的影响,获得了相应的铜氨络合物浓度及氨浸罐中液体的流速和压力分布等动力学过程。结果表明:改变氨浸罐出口和入口的位置可以明显影响铜氨络合物的生成浓度,氨从氨浸罐中部进入并从氨浸罐底部右下角流出速度大、氨浸效果好,氧化铜颗粒与氨反应充分,所获得的铜氨络合物浓度最高。研究成果对氨浸络合工艺装备设计具有重要指导意义。
Abstract:Based on the theory of multi-field coupling of porous media and the mass transfer of chemical reaction, the flow velocity, pressure and product concentration between copper oxide particles of ammonia in the process of ammonia leaching were studied by using Comsol Multiphysics simulation software. The model discussed in this paper set the inlet from the left, middle and right parts of the center of the tank, and the outlet from the right side and the middle of the bottom of the tank. The effects of different inlet and outlet positions of ammonia leaching tanks on the concentration of copper-ammonia complex during ammonia leaching were investigated. The corresponding copper ammonia complex concentration and the kinetics process of the flow velocity and pressure distribution of the liquid in the ammonia leaching tank were obtained. The results showed that the formation concentration of copper-ammonia complex could be obviously affected by changing the position of the inlet and outlet of ammonia leaching tank. The ammonia entering from the middle of the ammonia leaching tank and flowing out from the bottom right corner had a high velocity and the effect of ammonia leaching was good. Meanwhile, the copper oxide particles had a full reaction with ammonia, and the concentration of copper ammonia complex was the highest. The research results had important guiding significance for the equipment design of ammonia leaching complexation.
-
Key words:
- porous media /
- multi-field coupling /
- ammonia leaching /
- ammonia leaching tank /
- copper oxide ore
-

-
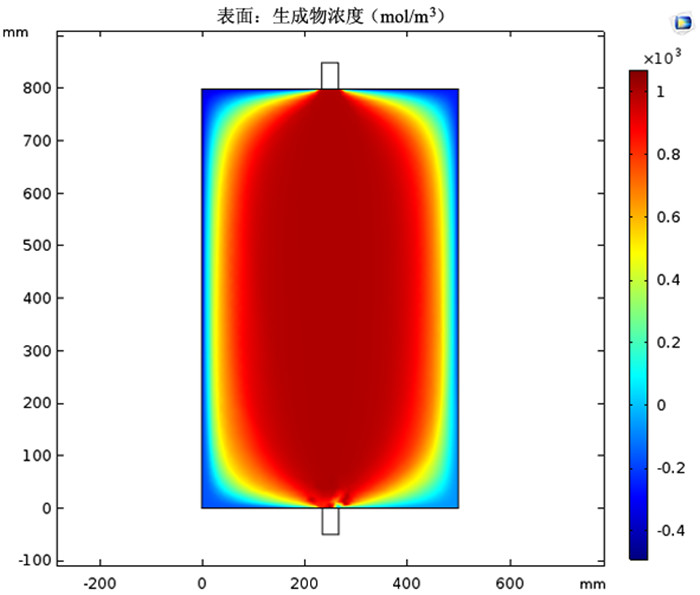
图 4 采用图 3(a)模型时的铜氨络合物浓度
Figure 4.
图 5 采用图 3(b)模型时的铜氨络合物浓度
Figure 5.
图 6 采用图 3(c)模型时的铜氨络合物浓度
Figure 6.
-
[1] 张纪辉.我国金属矿产资源全球化战略研究[D].北京: 中国人民大学, 2005.
http://d.wanfangdata.com.cn/Thesis/J0093559 [2] 孙肇均, 方维萱.我国有色金属矿山资源现状与新一轮找矿成就[J].中国金属通报, 2005, 36:2-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK200501870392
[3] 杨新生.氨浸过程浅析[J].有色金属, 1993(1):34-37. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000004881794
[4] 刘志雄.氨性溶液中含铜矿物浸出动力学及氧化铜-锌矿浸出工艺研究[D].长沙: 中南大学, 2012.
http://d.wanfangdata.com.cn/Thesis_Y2197996.aspx [5] 张铁民, 方建军, 蒋太国, 等.兰坪燕子洞含银氧化铜矿常温常压氨浸试验研究[J].矿产保护与利用, 2014(1):26-29. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=c190634d-4dfe-4dcf-830d-9b260b889d2d
[6] 蒋太国, 方建军, 张铁民, 等.氧化铜矿选矿技术研究进展[J].矿产保护与利用, 2014(2):49-53. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=a1ac81aa-08a1-4611-8d5f-f763fcfadb2f
[7] 方建军, 李艺芬, 鲁相林, 等.低品位氧化铜矿石常温常压氨浸工艺影响因素研究与工业应用结果[J].矿冶工程, 2008, 28(3):81-83. doi: 10.3969/j.issn.0253-6099.2008.03.021
[8] 郑永兴, 文书明, 刘健, 等.难处理氧化铜矿强化浸出的研究概况[J].矿产综合利用, 2011(2):33-36. doi: 10.3969/j.issn.1000-6532.2011.02.010
[9] 库建刚, 刘殿文, 张文彬.氧化铜矿氨浸渣的综合回收试验[J].中国有色冶金, 2007(4):30-32. doi: 10.3969/j.issn.1672-6103.2007.04.009
[10] 赵阳升.多孔介质多场耦合作用及其工程响应[M].北京:科学出版社, 2010.
-




 下载:
下载: