Flotation Study of Sulfur and Arsenic Reducing on Tungsten Ore Bearing High Sulfur and Arsenic
-
摘要:
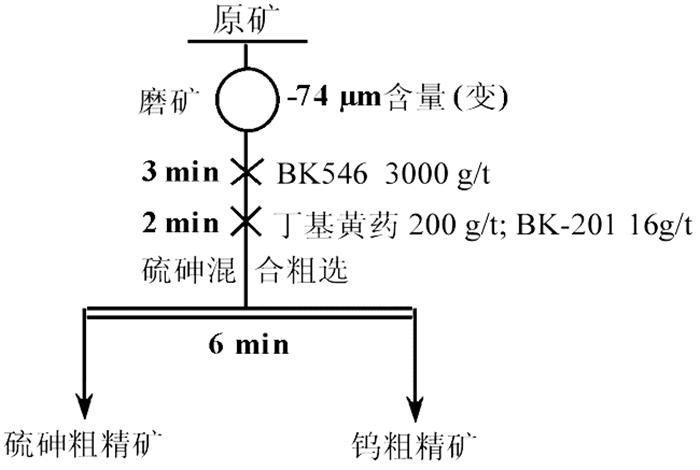
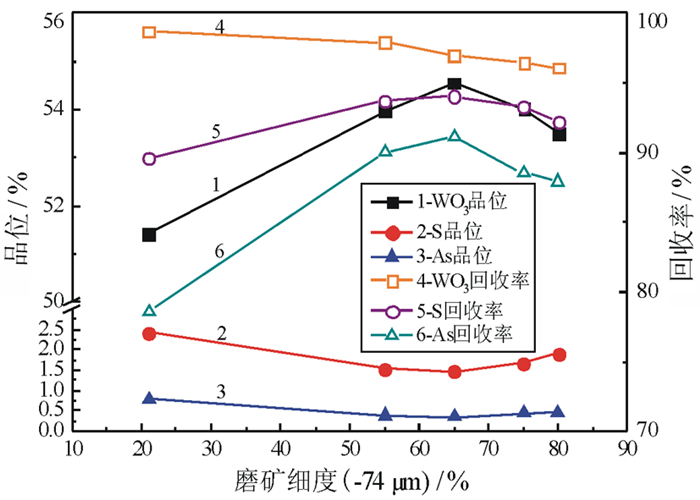
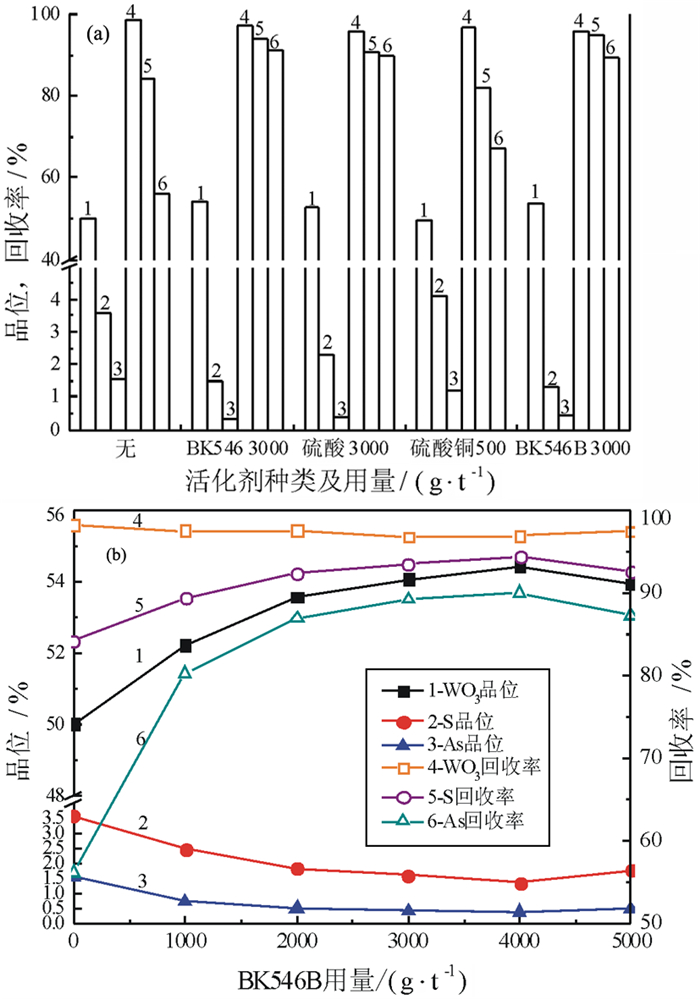
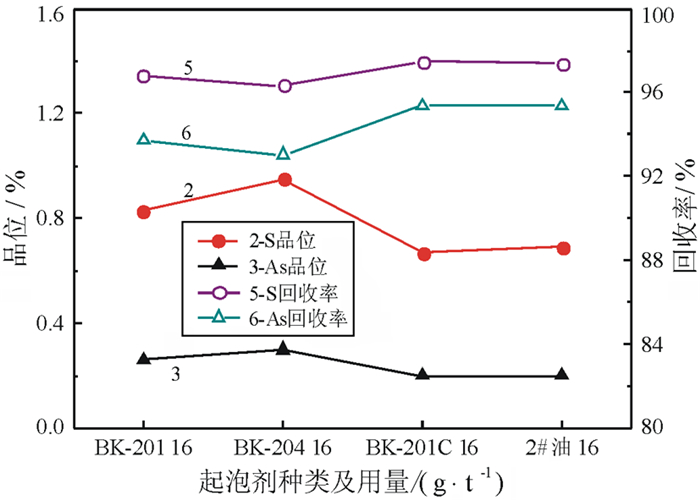
对某含Mo 0.55%、Bi 0.79%、Cu 0.66%、Zn 2.25%、S 15.95%、As 2.58%、WO3 35.84%的钨矿石进行了脱硫降砷浮选试验研究。该矿石由主干流程重选产出的-0.3 mm高含硫砷硫化物的细粒钨粗精矿。根据矿石的性质,采用硫砷混合浮选工艺流程。硫砷混合浮选时,采用高效的活化剂BK546B替代传统的硫酸,不仅有利于钨精矿中硫、砷杂质的脱除,更重要的是可改善因使用硫酸而造成的操作不便和不良的作业环境;采用选硫特效捕收剂AT608A与丁基黄药组合,有利于提高硫、砷的脱除率,并降低钨精矿中硫、砷杂质的含量,提高钨精矿品质。闭路试验获得含WO3 55.64%、含硫0.38%、含砷0.088%、WO3回收率为99.34%的钨精矿;而硫砷精矿中的WO3含量仅为0.66%,WO3在硫砷精矿中的损失率为0.66%。实现了钨精矿的高效脱硫降砷,并解决了困扰企业生产经营的难题。
Abstract:The flotation separation of tungsten ore with 0.55% Mo, 0.79% Bi, 0.66% Cu, 2.25% Zn, 15.95% S, 2.58% As and 35.84% WO3, which was obtained from trunk flowsheet with gravity concentration of a tungsten concentrator, was studied. According to the ore property, The sulfur-arsenic mixed flotation process was adopted. An efficient activator BK546B as a substitute for conventional sulfuric acid was used not only to improve the removal of sulfur and arsenic in tungsten concentrates, more important to improve the inconvenience of operation and unhealthy working environment caused by using sulfuric acid; the combined effective collector AT608A and butyl xanthate were used to remove sulfur and arsenic minerals from tungsten concentrates, and reduce the contents of sulfur and arsenic in tungsten concentrates and improve the quality of the tungsten concentrates. The results of the closed circuit test show that a tungsten concentrate can be obtained with WO3 grade 55.64%, S grade 0.38%, As grade 0.088% and WO3 recovery rate of 99.34%, and 0.66% of WO3 content and 0.66% of WO3 loss in the sulfur and arsenic concentrates are achieved separately. It can be achieved the effective removal of the sulfur and arsenic minerals from the tungsten ore by using the new process studied, and solve the difficulty of production and management.
-
Key words:
- wolframite /
- pyrite /
- arsenopyrite /
- fine grained /
- flotation separation /
- activator /
- combination of collectors
-

-
表 1 试样的主要化学成分分析结果 /%
Table 1. Analysis results of run-of-mine ore
WO3 Cu Mo S Bi As Pb Zn Ag Au 35.84 0.66 0.55 15.95 0.79 2.58 0.25 2.25 260 0.80 Fe P SiO2 Al2O3 K2O Na2O MgO CaO F C 15.69 0.64 4.41 0.79 0.36 0.18 0.74 11.90 2.45 0.34 注:Ag、Au单位为g·t-1。 表 2 矿石中硫的化学物相分析结果 /%
Table 2. Analysis results of sulfur phase of run-of-mine ore
相别 硫酸盐中硫 硫化物中硫 总硫 含量 0.099 16.280 16.379 分布率 0.60 99.40 100.00 表 3 矿石中砷的化学物相分析结果 /%
Table 3. Analysis results of arsenic phase of run-of-mine ore
相别 氧化砷中砷 硫化砷中砷 总砷 含量 0.000 2 2.590 0 2.590 2 分布率 0.008 99.992 100.000 表 4 闭路试验结果 /%
Table 4. Results of closed-circuit test
产品 产率 品位 回收率 WO3 S As WO3 S As 硫砷精矿 36.04 0.66 43.54 7.06 0.66 98.47 97.85 钨精矿 63.96 55.64 0.38 0.088 99.34 1.53 2.15 原矿 100.00 35.82 15.93 2.60 100.00 100.00 100.00 -
[1] 徐晓萍, 梁冬云, 王国生.广西某锑锌银钨多金属矿选矿工艺研究[J].有色金属(选矿部分), 2011(6):1-3. doi: 10.3969/j.issn.1671-9492.2011.06.001
[2] 谭欣, 王中明, 赵杰, 等.某铜钼钨矿石浮选分离试验研究[J].中国矿业, 2017, 26(3):117-121. doi: 10.3969/j.issn.1004-4051.2017.03.024
[3] 肖军辉, 樊珊萍, 王振, 等.湖北低品位钨钛多金属矿综合回收试验研究[J].稀有金属, 2013, 37(4):656-665. doi: 10.3969/j.issn.0258-7076.2013.04.022
[4] 方能香, 胡斌.钼、铋、铜、钨多金属矿石选矿试验研究[J].金属矿山, 2005(1):39-41. doi: 10.3321/j.issn:1001-1250.2005.01.011
[5] 谭欣, 王中明, 赵杰, 等.含钼、铜、锌、铋多金属硫化矿无氰分离工艺研究[J].稀有金属, 2015, 39(4):350-356. http://d.old.wanfangdata.com.cn/Periodical/xyjs201504009
[6] 刘日和.黑钨矿伴生硫化矿回收工艺改进[J].江西有色金属, 2005, 19(2):23-25. doi: 10.3969/j.issn.1674-9669.2005.02.007
[7] 宋振国, 孙传尧, 王中明, 等.中国钨矿选矿工艺现状及展望[J].矿冶, 2011, 20(1):1-7. http://d.old.wanfangdata.com.cn/Periodical/ky201101001
[8] 孙传尧, 程新朝, 李长根.钨铋钼萤石复杂多金属矿综合选矿新技术—柿竹园法[J].中国钨业, 2004, 19(5):8-13. doi: 10.3969/j.issn.1009-0622.2004.05.002
[9] 李贞.细粒钨精矿脱硫降砷工艺的技术改造与生产实践[J].有色金属科学与工程, 2011, 2(4):63-66. http://d.old.wanfangdata.com.cn/Periodical/jxysjs201104015
[10] 叶雪均, 张亚娟, 刘军.某钨锡矿枱浮中尾矿钼铜锌浮选试验研究[J].中国钨业, 2006, 21(6):23-24. doi: 10.3969/j.issn.1009-0622.2006.06.007
[11] 刘磊, 王双玉, 孙晓华, 等.青海某铁铜矿铜、硫综合回收试验研究[J].矿产保护与利用, 2017(6):52-56. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=89813dd1-9804-4248-a1e7-b58c55b0a76d
[12] 常永强, 付毅.越南某铜矿石选矿试验研究[J].矿产保护与利用, 2013(4):16-19. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=6ce3d08f-a584-47e3-bf48-8e7d5cd14f06
-



 下载:
下载: