Study on the Mechanism of Floatability Differences between Calcite and Wollastonite
-
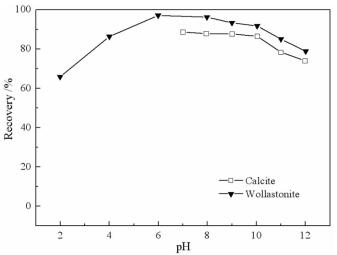
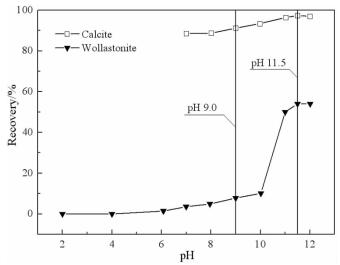
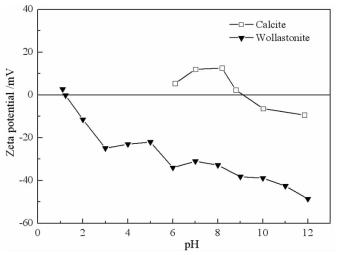
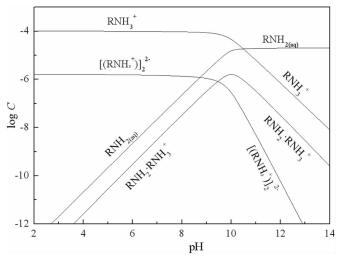
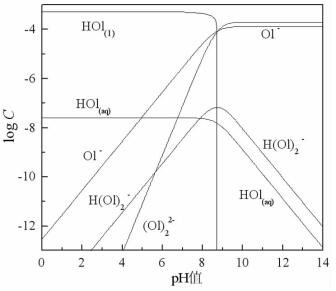
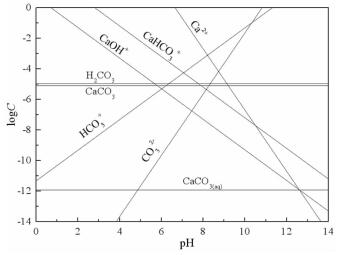
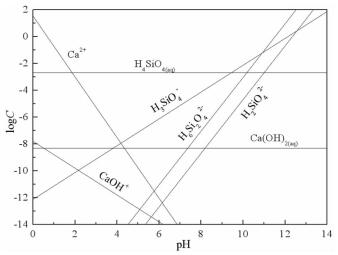
摘要: 方解石与硅灰石常相互伴生,因矿物晶格中都含有Ca2+而具有一些相似的表面性质和溶解特性;但是,两种矿物所含阴离子基团的不同,又使它们在浮选中表现出一定的差异性。本文以方解石和硅灰石为研究对象,分别研究了十二胺和油酸钠对方解石和硅灰石浮选行为的影响;通过Zeta电位分析和logC-pH分析,研究了不同pH值条件下方解石和硅灰石的表面荷电情况以及溶液中的优势组分情况;通过XPS测试和MS模拟,研究了方解石和硅灰石表面Ca质点密度和Ca不饱和键密度的情况。Zeta电位分析和logC-pH分析的结果表明,由于定位离子的不同,方解石比硅灰石带有更多的表面净剩正电荷;XPS和MS模拟的研究结果则表明,方解石比硅灰石具有更多的表面Ca质点密度和Ca不饱和键密度。Abstract: Calcite and wollastonite are often associated with each other in ore. For the same Ca2+ contained in the mineral lattices, some similar surface characteristics and dissolution characteristics are shown in the two minerals. But for the existence of the different anionic groups in their mineral lattices, some differences are shown in the flotations of the two minerals. In this paper, the floatability differences of calcite and wollastonite were studied by the single mineral test when lauryl amine and sodium oleate were used as collectors. The surface potentials and the chief components of calcite and wollastonite in solutions under different pH values were studied by zeta potential analysis and logC-pH analysis of the two minerals. The density of calcium exposure and the density of unsaturated bonds of calcium on the surfaces of calcite and wollastonite were studied by XPS analysis and MS simulations. The results of zeta potential and logC-pH analysis showed that the surface potentials of calcite were more than wollastonite due to the different locating ions of the two minerals; XPS and MS simulation results showed that calcite had more density of calcium exposure and the density of unsaturated bonds of calcium than wollastonite.
-
Key words:
- calcite /
- wollastonite /
- floatability differences /
- crystal chemistry
-

-
表 1 方解石与硅灰石元素的相对含量
Table 1. Relative content of elements in calcite and wollastonite
Sample pH Relative content /% Ca 2p2/3 O 1s C 1s Si 2p CaCO3 pH=9.0 15.54 39.67 18.89 — pH=11.5 16.00 43.05 16.09 — CaSiO3 pH=9.0 11.34 46.45 — 18.69 pH=11.5 11.60 43.75 — 19.77 表 2 方解石与硅灰石元素的原子轨道结合能
Table 2. Binding energy of elements in calcite and wollastonite
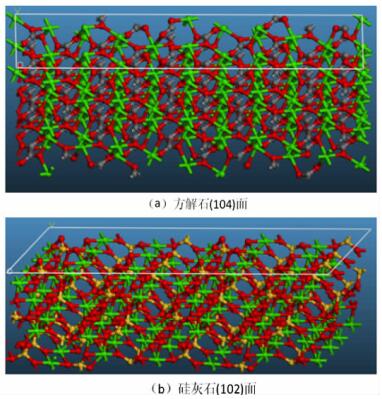
Sample pH Binding energy/eV Ca 2p2/3 O 1s C 1s Si 2p CaCO3 pH=9.0 346.59 531.17 286.54 — pH=11.5 346.34 530.90 288.88 — CaSiO3 pH=9.0 346.92 531.89 — 101.96 pH=11.5 346.68 531.80 — 101.90 表 3 方解石和硅灰石主要解离面上的Ca质点密度和Ca不饱和键密度
Table 3. Densities and unsaturated bonds of calcium on the main cleavage planes of calcite and wollastonite
Mineral Cleavage plane Chemical formula Area /nm2 Number (Ca) Density (Ca) /nm2 Number (u-Ca) Density (u-Ca) /nm2 Calcite (104) Ca96C96O288 4.69 20 4.26 34 7.24 Wollastonite (102) Ca96Si96O288 5.58 16 2.87 16 2.87 -
[1] 王景阳, 蒋志勇, 童振声.硅灰矿石中的硅灰石、方解石、白云石的物相分析法——钙量法[J].吉林地质, 1982(1):65-73.
[2] 王秀文.硅灰石与方解石、石英的浮选分离(摘要)[J].国外金属矿选矿, 1986(9):26-27.
[3] 毛钜凡, 程卫泉.硅灰石与方解石的浮选分离研究[J].矿冶工程, 1991, 11(2):43-47.
[4] 袁继祖.硅灰石与方解石、石英、长石浮选分离的探讨[C].苏州: 中国硅酸盐协会, 1988.
[5] 孙传尧, 印万忠.硅酸盐矿物浮选原理[M].北京:科学出版社, 2001.
[6] HUANG FUGEN, R SIVAMOHAN. Behaviour of different particle size fractions in the flotation of calcite from wollastonite and microcline[J]. Minerals Engineering, 1990, 3(3-4):257-271. doi: 10.1016/0892-6875(90)90121-Q
[7] 高志勇.三种含钙矿物晶体各向异性与浮选行为关系的基础研究[D].长沙: 中南大学, 2013.
[8] HJ MONKHORST, JD PARK. Special points for Brillouin-zone integrations[J]. Physical Review B, 1976, 13(12):5188-5192. doi: 10.1103/PhysRevB.13.5188
[9] 王淀佐, 胡岳华.浮选溶液化学[M].长沙:湖南科学技术出版社, 1988.
[10] 胡岳华, 徐竞, 罗超奇, 等.菱锌矿/方解石胺浮选溶液化学研究[J].中南工业大学学报, 1995, 26(5). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199500834215
[11] 胡岳华, 王淀佐.脂肪酸钠浮选盐类矿物的作用机理研究[J].矿冶工程, 1990(2):20-23.
[12] 赵古田.固液界面双电层结构的理论与实验研究[D].长沙: 中南大学, 2014.
[13] 王淀佐, 胡岳华.氢氧化物表面沉淀在石英浮选中的作用[J].中南矿冶学院学报, 1990, 21(6):248-253. http://www.cnki.com.cn/Article/CJFDTotal-ZNGD199003003.htm
[14] 王淀佐, 胡岳华.金属离子在氧化物矿物/水界面的吸附及浮选活化机理[J].中南矿冶学院学报, 1987, 18(5):501-508.
[15] TA CLARKE, EN RIZKALLA. X-ray photoelectron spectroscopy of some silicates[J]. Chemical Physics Letters, 1976, 37(3):523-526. doi: 10.1016/0009-2614(76)85029-4
[16] G HOLLINGER, FJ HIMPSEL. Probing the transition layer at the SiO2-Si interface using core level photoemission[J]. Applied Physics Letters, 1984, 44(1):93-95. doi: 10.1063/1.94565
[17] ML MILLER, PW LINTON. X-ray photoelectron spectroscopy of thermally treated silica (SiO2) surfaces[J]. Analytical Chemistry, 1985, 57(12):2314-2319. doi: 10.1021/ac00289a033
[18] T SUGAMA, LE KUKACKA, N CARCIELLO, et al. Study of interactions at water-soluble polymer/Ca(OH)2 or gibbsite interfaces by XPS[J]. Cement and Concrete Research, 1989, 19(6):857-867. doi: 10.1016/0008-8846(89)90098-7
[19] 韩海生.新型金属配合物捕收剂在钨矿浮选中的应用及其作用机理研究[D].长沙: 中南大学, 2017.
[20] 王力.气流磨制备高长径比硅灰石的技术研究[D].沈阳: 东北大学, 2005.
[21] 杨怡, 孙传敏, 龚夏生.硅灰石粉碎机理的研究[J].成都理工学院学报, 1998, 25(3):443-446.
-



 下载:
下载: