Preparation of Ba3(VO4)2 by Desorption and Precipitation Recovery of Vanadium in Chelating Resin
-
摘要:
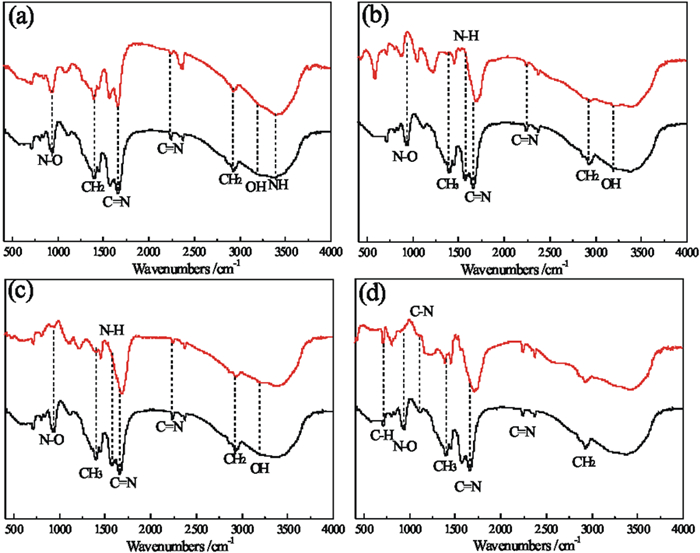
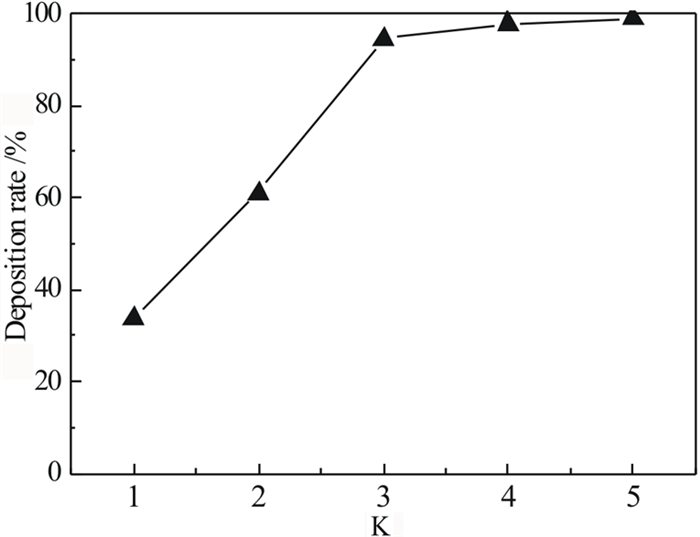
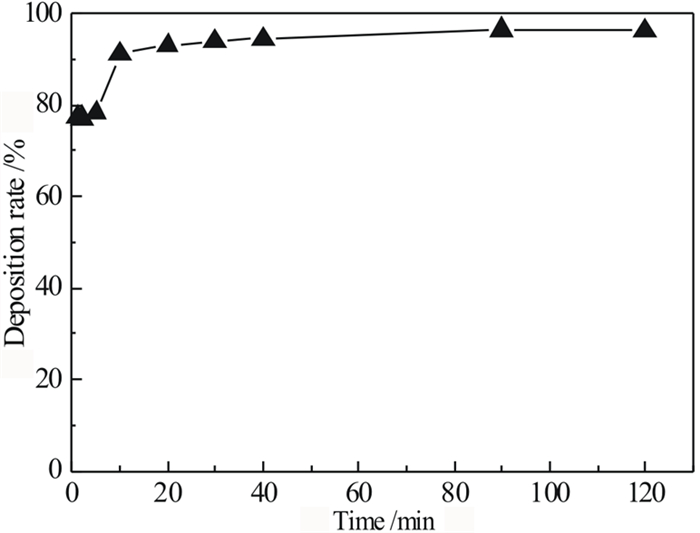
针对偕胺肟螯合树脂吸附回收拜耳循环母液中镓时钒发生共吸附且钒难解吸,导致树脂对镓的吸附能力降低的问题,研究了解吸剂种类、解吸剂浓度、解吸温度和解吸时间等因素对钒解吸率的影响,并对强碱解吸溶液中的钒离子沉淀回收制备钒酸钡进行了研究。试验结果显示:(1)氢氧化钠不会破坏树脂结构且能够解吸部分钒,当使用12 mol/L氢氧化钠在50℃的条件下解吸10 h,其解吸率可达到43.68%;(2)当分别使用10 mol/L盐酸、硝酸、硫酸时,钒解吸率高达60%~70%,但红外光谱测试结果显示树脂基团遭受破坏,可知强酸溶液会破坏树脂结构;(3)BaO对氢氧化钠溶液中的钒离子具有良好的沉淀效果;当K(BaO与溶液中V2O5的质量比)为3时,温度为室温,NaOH浓度为1 mol/L,沉淀时间为30 min时,钒的沉淀率可达到94.42%,其沉淀产物为Ba3(VO4)2。
Abstract:In the process of recovering gallium from Bayer circulating mother liquor by amidoxime chelating resin, gallium and vanadium co-adsorbed and was difficult to desorption, resulting in the adsorption capacity of gallium on resin is reduced. The effects of different desorption agents, concentration of desorption agents, desorption temperature and desorption time on desorption rate were studied, and the preparation of barium vanadate by precipitation and recovery of vanadium ions from strong alkali desorption solution was studied. The results show that: (1) Desorption experiments show that sodium hydroxide does not destroy the resin structure and has a certain desorption rate. When the resin was desorbed by using 12 mol/L sodium hydroxide at 50 ℃ for 10 hours, the desorption rate reaches 43.68%; (2) When 10 mol/L hydrochloric acid, nitric acid and sulfuric acid are used respectively, the desorption rate of vanadium is as high as 60%~70%, but the infrared spectrum test results show that the resin group is damaged. It can be known that 10mol/L strong acid solution will destroy the resin structure. (3) BaO has good precipitation effect on vanadium ion in sodium hydroxide solution; when K (mass ratio of BaO to V2O5 in solution) is 3, the temperature is room temperature, NaOH concentration is 1 mol/L, and precipitation time is 30 min, the precipitation rate of vanadium can reach 94.42%, and the precipitation product is Ba3 (VO4)2.
-
Key words:
- amidoxime chelating resin /
- Vanadium /
- desorption /
- deposition
-

-
[1] 翟秀静, 吕子剑.镓冶金[M].北京:冶金工业出版社, 2010.
[2] 赵汀, 秦鹏珍, 王安建等.镓矿资源需求趋势分析与中国镓产业发展思考[J].地球学报, 2017, 38(1):77-84. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqxb201701012
[3] 赵秦生, 李中军.钒冶金[M].长沙:中南大学出版社, 2010.
[4] 段国义.富集-电解法回收氧化铝循环母液中镓的电流效率及杂质影响问题研究[D].北京: 北京化工大学, 2016.
http://cdmd.cnki.com.cn/Article/CDMD-10010-1016322246.htm [5] BAUTISTA, R. G. Processing to obtain high-purity gallium[J]. JOM, 2003, 55(3):23-26. doi: 10.1007/s11837-003-0155-2
[6] ZHAO Z, LI X H, CHAI Y Q, et al. Adsorption performances and mechanisms of amidoxime resin toward gallium(Ⅲ) and vanadium(V) from bayer liquor[J]. ACS Sustainable Chemistry & Engineering, 2015, 4(1):53-59.
[7] 郑琦, 韦悦周, 何春林, 等.广西某铝母液中镓钒铝的偕胺肟螯合树脂吸附与解吸试验[J].金属矿山, 2019, V48(3):111-115. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jsks201903019
[8] 李延芬, 刘叶凤, 罗浩, 等.提钒技术研究进展[J].化工进展, 2016, 35(S1):223-229. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz2016z1037
[9] 陈肖虎, 陈颖, 甘蔓, 等.沉淀法从拜耳法铝酸钠溶液中分离钒[J].过程工程学报, 2010, 10(S1):142-145. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgyj2010z1029
[10] 熊巍, 林树坤, 谢勇平.液相法合成Ba3(VO4)2晶体原料[J].福州大学学报(自然科学版), 2003, 31(5):618-621. doi: 10.3969/j.issn.1000-2243.2003.05.026
[11] 周振泽, 张德, 徐建梅, 等.Ba3(VO4)2合成过程的研究[J].材料工程, 2009(11):20-22. doi: 10.3969/j.issn.1001-4381.2009.11.005
[12] ZHANG Z, ZHU H, LI Y, et al. Low temperature sintering and dielectric properties of Ba3(VO4)2 microwave ceramics using CO2O3 additives[J]. Journal of Materials Science:Materials in Electronics, 2017, 28(24):18474-18479. doi: 10.1007/s10854-017-7794-5
[13] KHATRI P, BEHERA B, SRINIVAS V, et al. Structural and dielectric properties of Ba3V2O8 ceramics. Current Applied Physics, 2009, 9(2):515-519. doi: 10.1016/j.cap.2008.05.002
[14] 蒙杰杰, 何春林, 李杰, 等.拜耳循环母液镓吸附-萃取富集试验[J].矿产保护与利用, 2020(1):110-117. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=8092b6ed-dc4d-4f95-b349-4e3c585a49df
-




 下载:
下载: