The Action Mechanism of Ca-oleate Colloid Collector During the Flotation of Calcium-Containing Minerals
-
摘要:
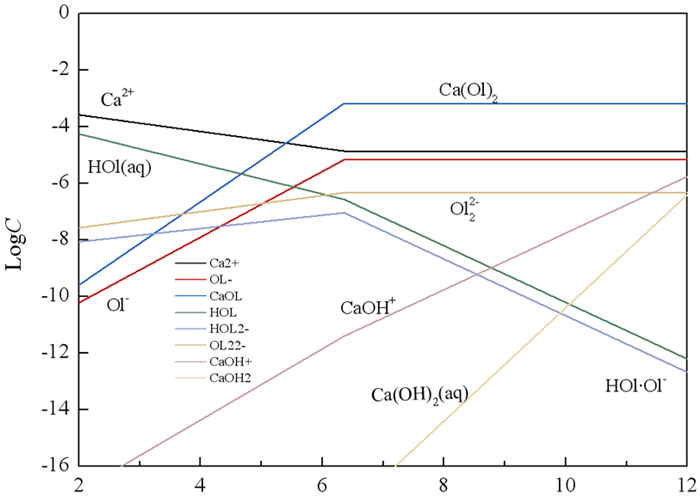
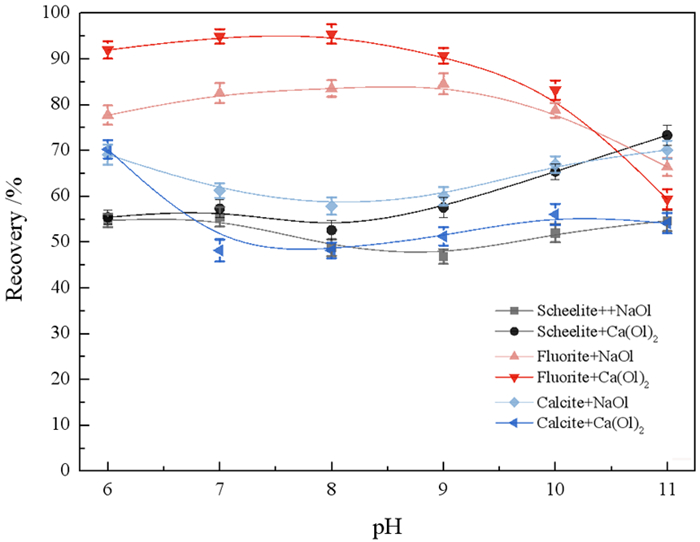
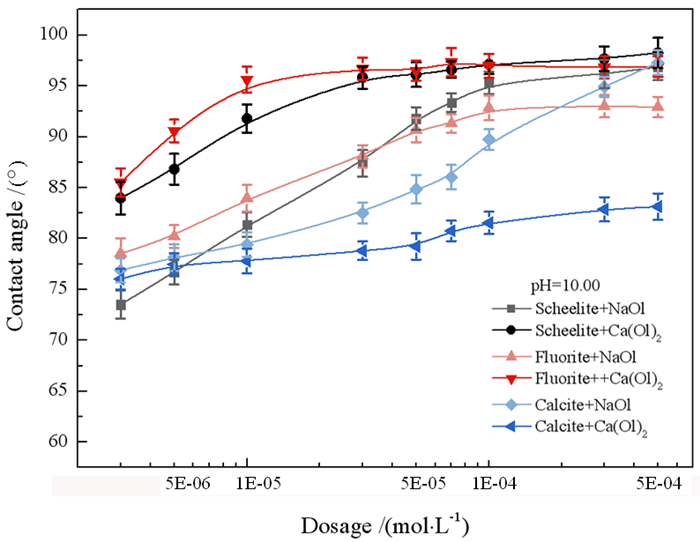
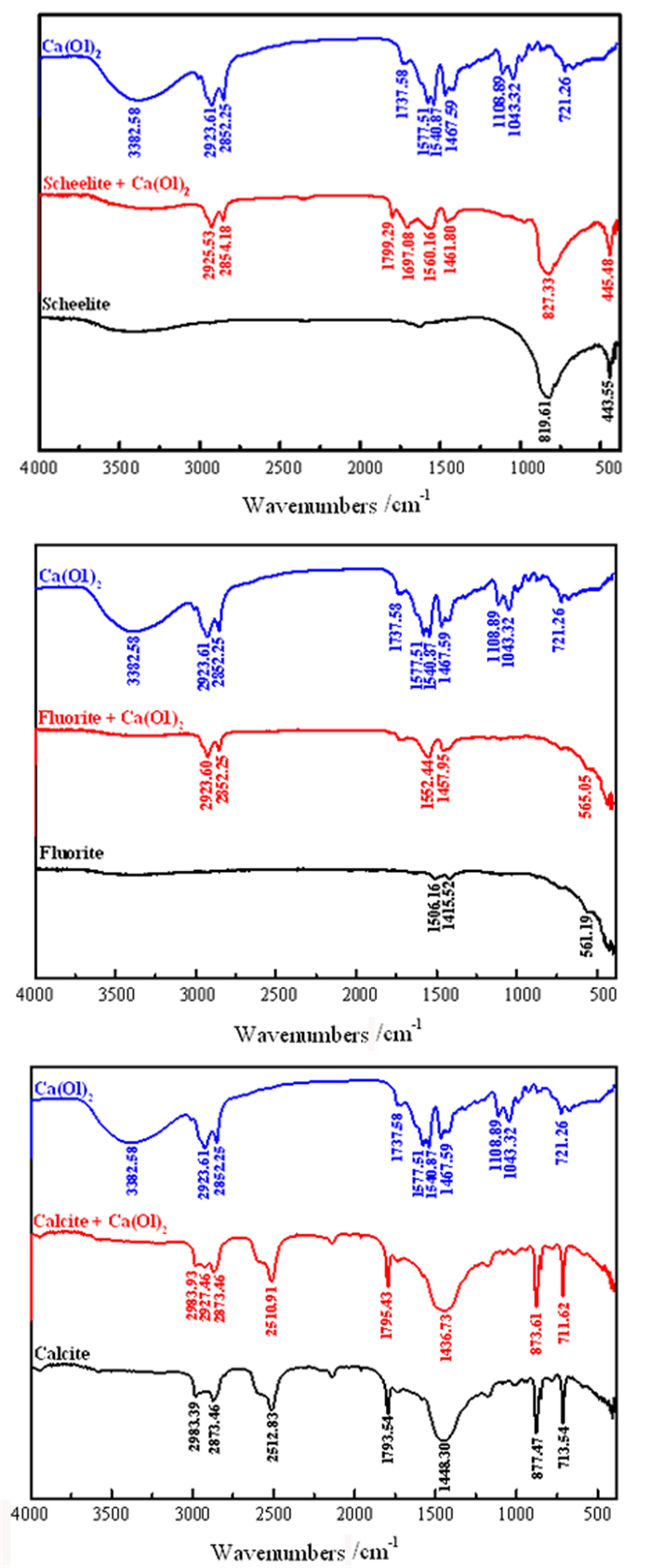
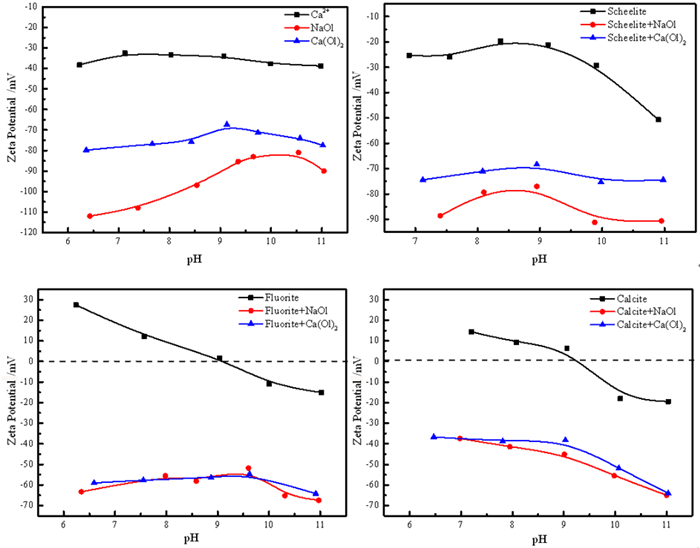
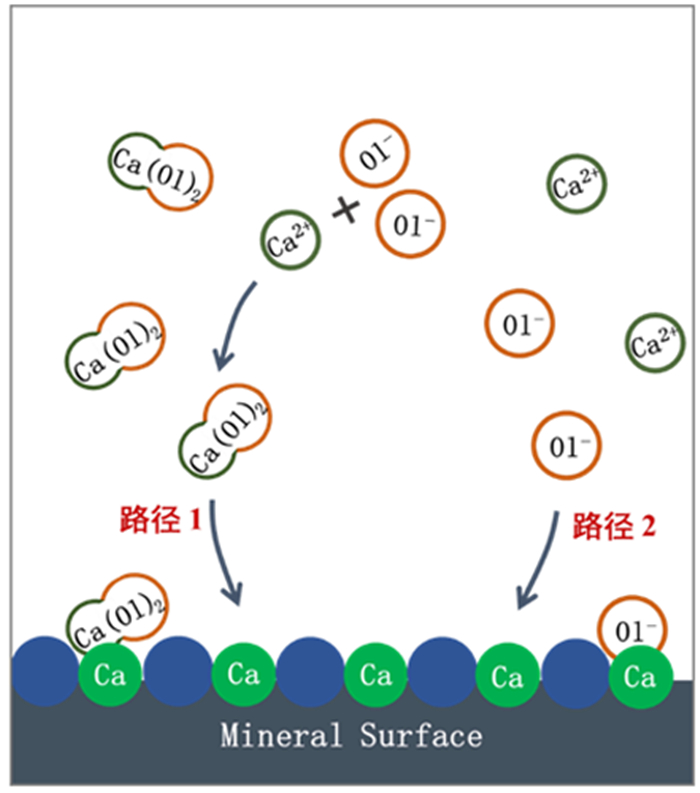
研究了油酸钠浮选白钨矿、萤石和方解石过程中Ca-油酸[Ca(OL)2]胶体的存在及其对浮选的影响。溶液化学计算表明,三种含钙矿物溶解的钙离子可以与油酸阴离子反应生成Ca(OL)2胶体,并且在碱性条件下作为溶液中的主要组分存在和发挥作用。纯矿物试验结果表明,Ca(OL)2胶体对白钨矿和萤石的捕收能力较油酸钠强,对方解石的捕收能力较油酸钠弱。Ca(OL)2胶体吸附后的三种矿物表面疏水性差异增大,白钨矿、萤石表面疏水性强于油酸钠作用后,而方解石表面疏水性弱于油酸钠作用后。Ca(OL)2胶体在白钨矿表面发生化学吸附,在萤石和方解石表面以化学吸附为主,当pH < 9.0时存在一定的静电吸附作用。油酸钠浮选含钙矿物过程中除油酸阴离子直接作用于矿物表面的路径外,存在另外一种重要的作用路径:钙离子与油酸阴离子在溶液中首先生成Ca(OL)2胶体,Ca(OL)2胶体在溶液中迁移至矿物表面发生吸附。
Abstract:The existence of Ca-oleate (Ca(OL)2) colloid and its effect on flotation during the flotation of scheelite, fluorite and calcite with sodium oleate were studied. Solution chemistry showed that the dissolved calcium ions from the three calcium-containing minerals can react with oleate anions to form Ca(OL)2 colloids, which exist and function as the main components in solutions under alkaline conditions. The pure minerals tests showed that the collection ability of Ca(OL)2 colloid for scheelite and fluorite was stronger than that of sodium oleate, and for calcite was weaker than that of sodium oleate. The difference in the surface hydrophobicity of the three minerals after Ca(OL)2 colloid adsorption increased. The surface hydrophobicity of scheelite and fluorite was stronger than that of sodium oleate, while the surface hydrophobicity of calcite was weaker than that of sodium oleate. Chemical adsorption of Ca(OL)2 colloids occurred on the surface of scheelite. On the surface of fluorite and calcite, it was mainly chemical adsorption, and there was also electrostatic adsorption when pH < 9.0. In the flotation process of calcium-containing minerals with sodium oleate, in addition to the direct action of oleic acid anions on the surface of minerals, there was another important action path: calcium ions and oleic acid anions first generate Ca(OL)2 colloids in solution, and Ca(OL)2 colloids migrated to the mineral surface in solution and adsorbed on the minerals surface.
-
Key words:
- Ca(OL)2 colloid /
- scheelite /
- fluorite /
- calcite /
- flotation
-

-
表 1 钙离子存在下,油酸钠溶液中的反应及反应平衡常数[9, 17]
Table 1. Solution chemistry balance expressions and balance constants of sodium oleate in solution chemistry calculation in the presence of calcium ion
反应式 logK Ca2+OH- $ \rightleftharpoons $ CaOH+2.51×10 OH-CaOH+ $ \rightleftharpoons $ Ca(OH)2(aq)2.34×101 OHl(aq) $ \rightleftharpoons $ Ol-+H+1.10×10-5 2Ol-H+ $ \rightleftharpoons $ HOl·Ol-7.94×109 2Ol- $ \rightleftharpoons $ (Ol)22-1.00×104 H2O $ \rightleftharpoons $ H++OH-1.00×1014 Ca2++2OH- $ \rightleftharpoons $ 2Ca(OL)23.00×10-16 Ol-+H+ $ \rightleftharpoons $ HOl2.82×10-13 -
[1] 刘红尾, 许增光. 石灰法常温浮选低品位白钨矿的工艺研究[J]. 矿产综合利用, 2013(2): 33-35. doi: 10.3969/j.issn.1000-6532.2013.02.009
LIU H W, XU Z G. Technology research on flotation of low-grade scheelite at normal temperature by lime-based method[J]. Multipurpose Utilization of Mineral Resources, 2013(2): 33-35 doi: 10.3969/j.issn.1000-6532.2013.02.009
[2] 杨子轩, 谢贤, 童雄, 等. 石灰在浮选过程中的作用[J]. 矿产综合利用, 2015(2): 17-21. doi: 10.3969/j.issn.1000-6532.2015.02.003
YANG Z X, XIE X TONG X, et al. Research on the effect of lime in flotation process[J]. Multipurpose Utilization of Mineral Resources, 2015(2): 17-21. doi: 10.3969/j.issn.1000-6532.2015.02.003
[3] 王建军, 高志勇, 孙伟, 等. 白钨矿常温浮选基础研究[J]. 金属矿山, 2016(2): 66-71. doi: 10.3969/j.issn.1001-1250.2016.02.014
WANG J J, GAO Z Y, SUN W, et al. Basic research of scheelite flotation at normal temperature[J]. Metal Mine, 2016(2): 66-71. doi: 10.3969/j.issn.1001-1250.2016.02.014
[4] 刘清高, 韩兆元, 管则皋. 白钨矿浮选研究进展[J]. 中国钨业, 2009, 24(4): 23-27. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU200904009.htm
LIU Q G, HAN Z Y, GUAN Z G. Research progress on scheelite flotation technology[J]. China Tungsten Industry, 2009, 24(4): 23-27. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU200904009.htm
[5] 刘红尾. 难处理白钨矿常温浮选新工艺研究[D]. 长沙: 中南大学, 2010.
LIU H W. Study on a new technology for refractory scheelite flotation at room temperature[D]. Changsha: Central South University, 2010.
[6] GAN J D, HU Y H, SUN W, et al. Enhanced separation of fluorite from calcite in acidic condition[J]. Minerals Engineering, 2019, 133: 103-105. doi: 10.1016/j.mineng.2019.01.013
[7] HAN H S, PENG M S, HU Y H, et al. An SFG spectroscopy study of the interfacial water structure and the adsorption of sodium silicate at the fluorite and silica surfaces[J]. Minerals Engineering, 2019, 138: 178-187. doi: 10.1016/j.mineng.2019.04.028
[8] SUN W, HAN H, HU Y, et al. Flotation theory and research progress of metal ion coordination regulation molecule assembly[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 927-941.
[9] FREE M L, MILLER J D. The significance of collector colloid adsorption phenomena in the fluorite/oleate flotation system as revealed by FTIR/IRS and solution chemistry analysis[J]. International Journal of Mineral Processing, 1996, 48(3): 197-216.
[10] ANTTI B M, FORSSBERG E. Pulp chemistry in industrial mineral flotation. Studies of surface complex on calcite and apatite surfaces using FTIR spectroscopy[J]. Minerals Engineering, 1989, 2(2): 217-227. doi: 10.1016/0892-6875(89)90042-3
[11] FUERSTENAU M C, CUMMINS W F. The role of basic aqueous complexes in anionic flotation of quartz[J]. Trans. Aime, 1967, 238: 196.
[12] ANANTHAPADMANABHAN K P, SOMASUNDARAN P. Surface precipitation of inorganics and surfactants and its role in adsorption and flotation[J]. Colloids and Surfaces, 1985, 13: 151-167. doi: 10.1016/0166-6622(85)80014-7
[13] RUTLAND M, PUGH R J. Calcium soaps in flotation deinking; fundamental studies using surface force and coagulation techniques[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1997, 125(1): 33-46.
[14] BROWN S C, RABINOVICH Y I, Moudgil B M. Calcium activation of silica surfaces with sodium oleate collector[J]. Mining, Metallurgy & Exploration, 2004, 21(3): 164-168.
[15] FA K, NGUYEN A V, Miller J D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation[J]. International Journal of Mineral Processing, 2006, 81(3): 166-177. doi: 10.1016/j.minpro.2006.08.006
[16] FA K Q, TAO J A, NALASKOWSKI J, et al. Interaction forces between a calcium dioleate sphere and calcite/fluorite surfaces and their significance in flotation[J]. Langmuir, 2003, 19(25): 10523-10530. doi: 10.1021/la035335j
[17] SUN W J, HAN H S, SUN W, et al. Novel insights into the role of colloidal calcium dioleate in the flotation of calcium minerals[J]. Minerals Engineering, 2022, 175.
[18] BECRAFT K A, MOOREB F G, RICHMOND G L. In-situ spectroscopic investigations of surfactant adsorption and water structure at the CaF2/aqueous solution interface[J]. Physical Chemistry Chemical Physics, 2004, 6(8): 1880-1889. doi: 10.1039/B313513F
[19] WANG R L, HAN H S, SUN W, et al. Hydrophobic behavior of fluorite surface in strongly alkaline solution and its application in flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 609.
[20] 李昀芮. 油酸铁的制备及其在稠油催化降黏中的应用[D]. 开封: 河南大学, 2018.
LI Y R. Preparation of ferric oleate and its application in catalytic aquathermolysis of heavy crude oil[D]. Kaifeng: Henan University, 2018.
[21] 陈德恒. 有机结构分析[M]. 北京: 科技出版社, 1985.
CHEN D H. Organic Structure Analysis[M]. Beijing: Science Press, 1985.
[22] 韩英, 钟康年. 含钙矿物浮选中的本体沉淀及其对矿物可浮性的影响[J]. 武汉化工学院学报, 1992(S1): 1-9. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG1992S1000.htm
HAN Y, ZHONG K N. Bulk precipitation and its effect on mineral flotability in flotation of the ores containing calcium. Journal of Wuhan Institute of Chemical Technology, 1992(S1): 1-9. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG1992S1000.htm
-




 下载:
下载: