Mechanism of Inhibitor EDTA in Flotation Separation of Niobite and Quartz
-
摘要:
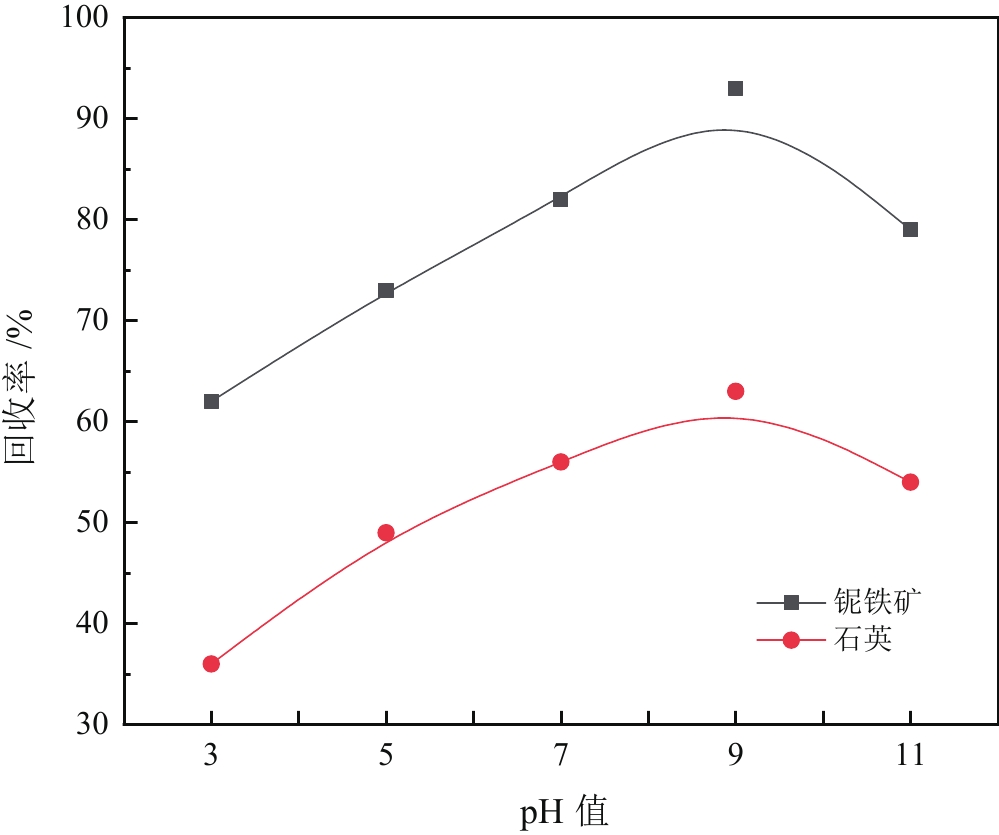
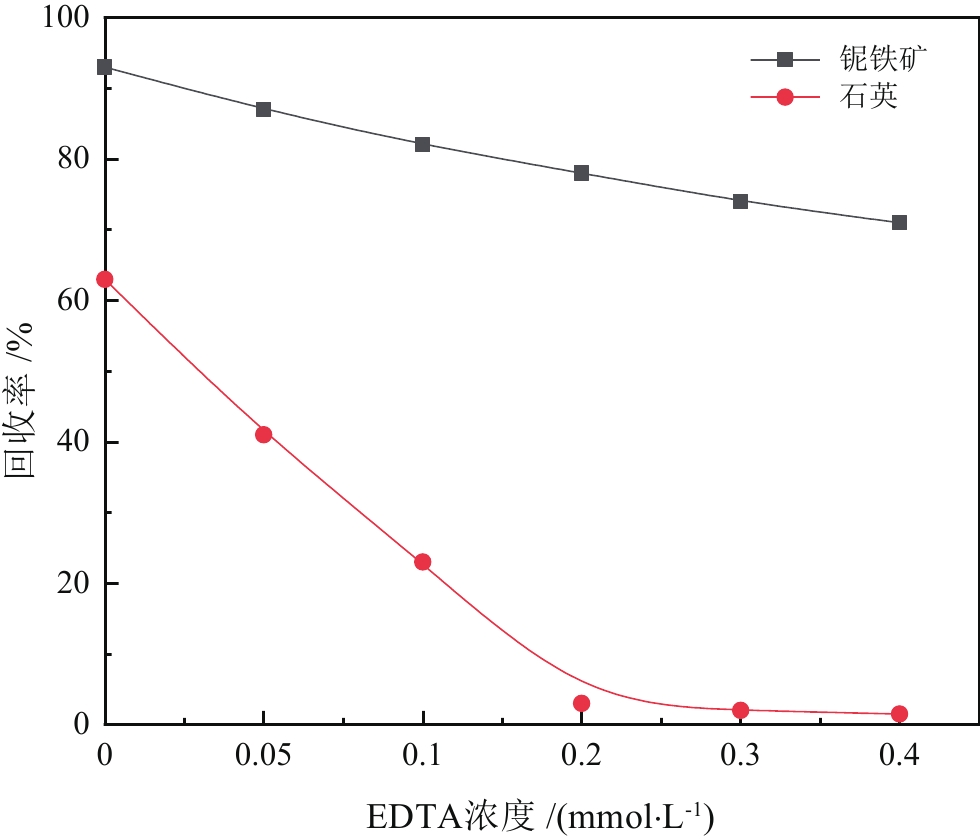
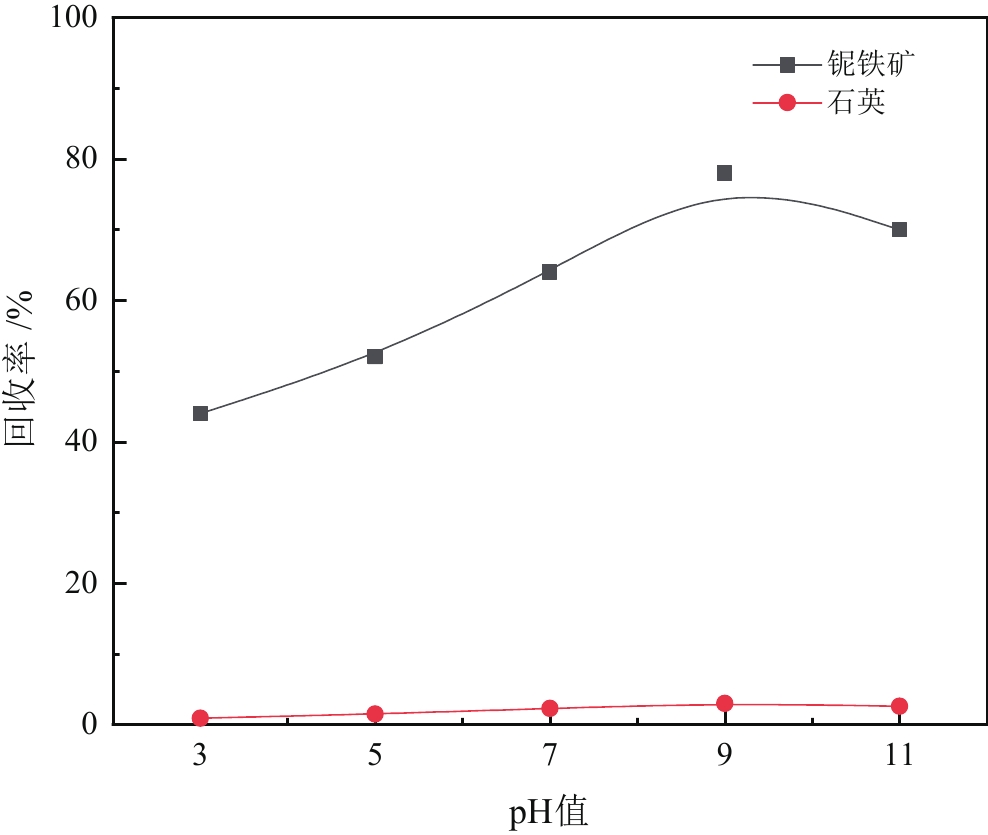
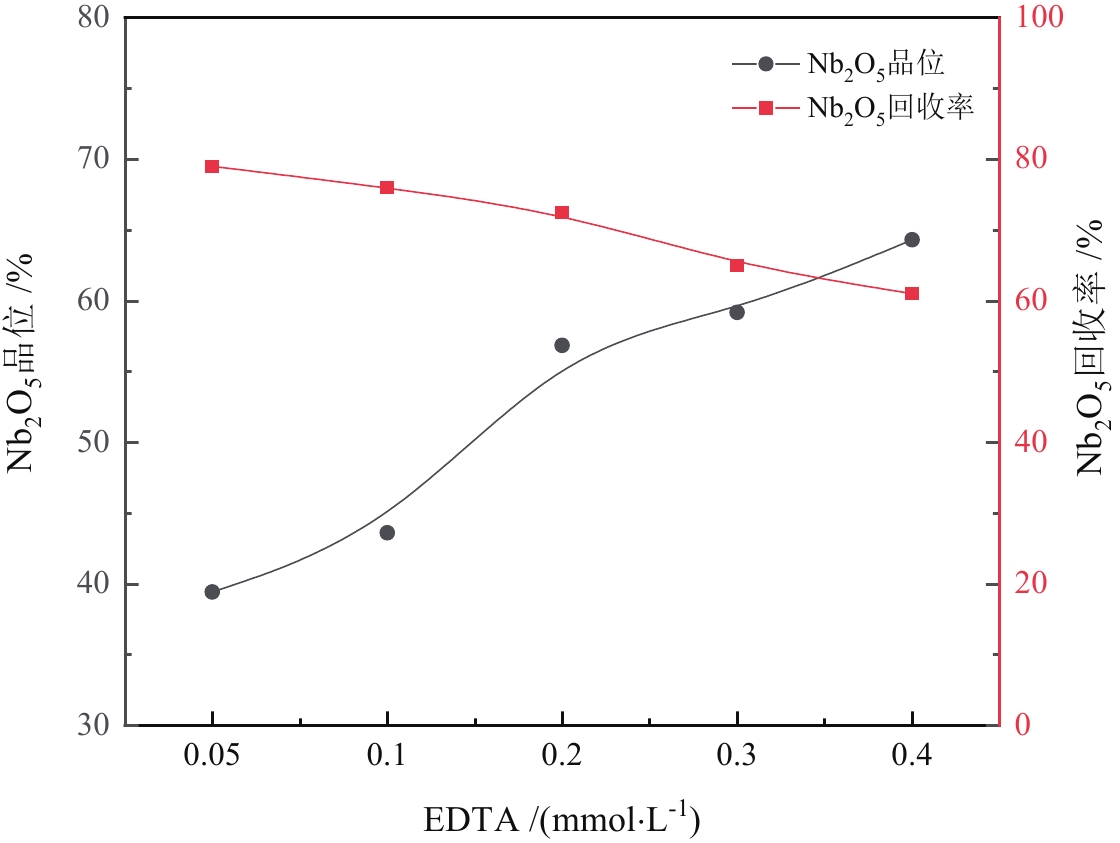
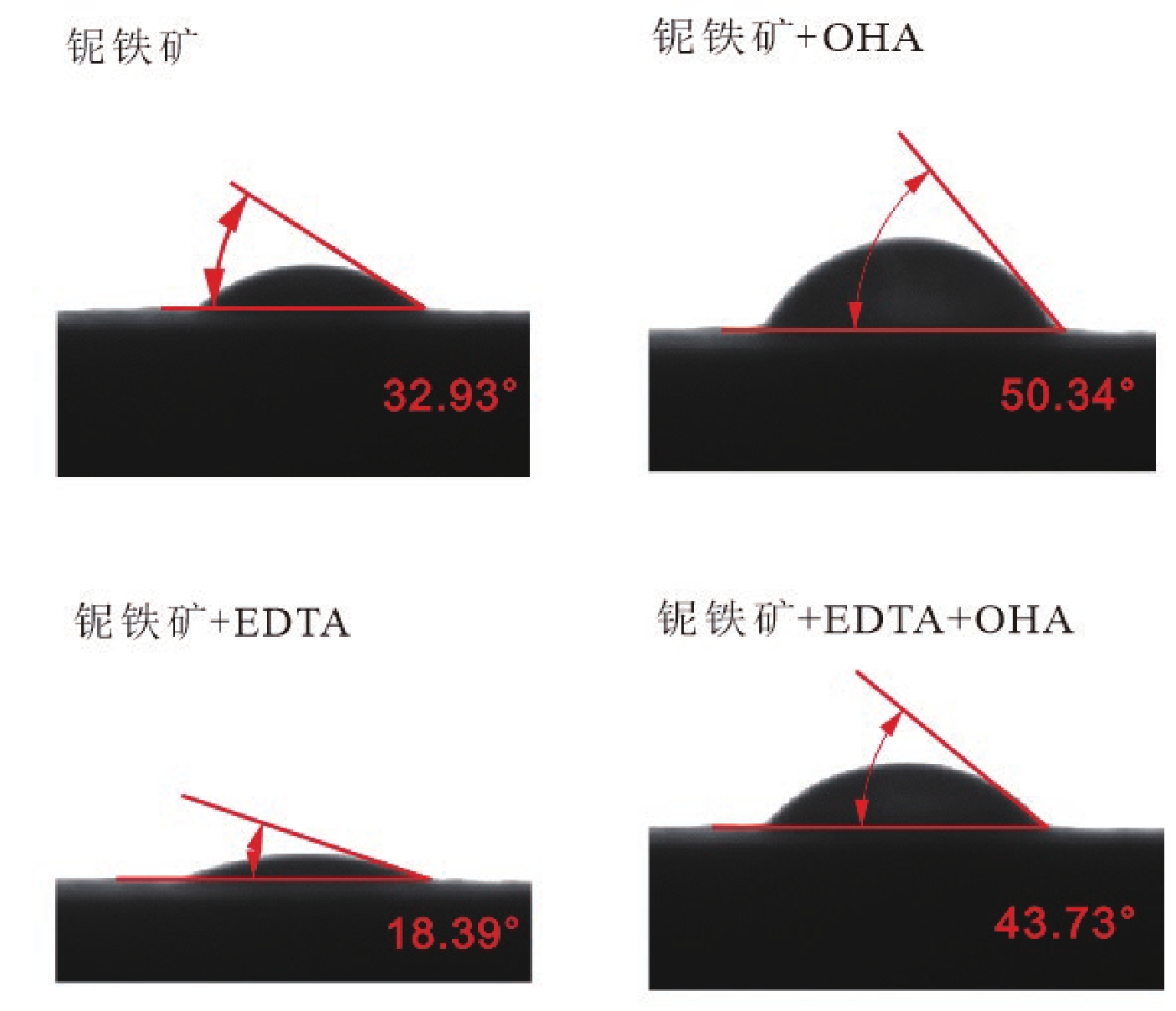
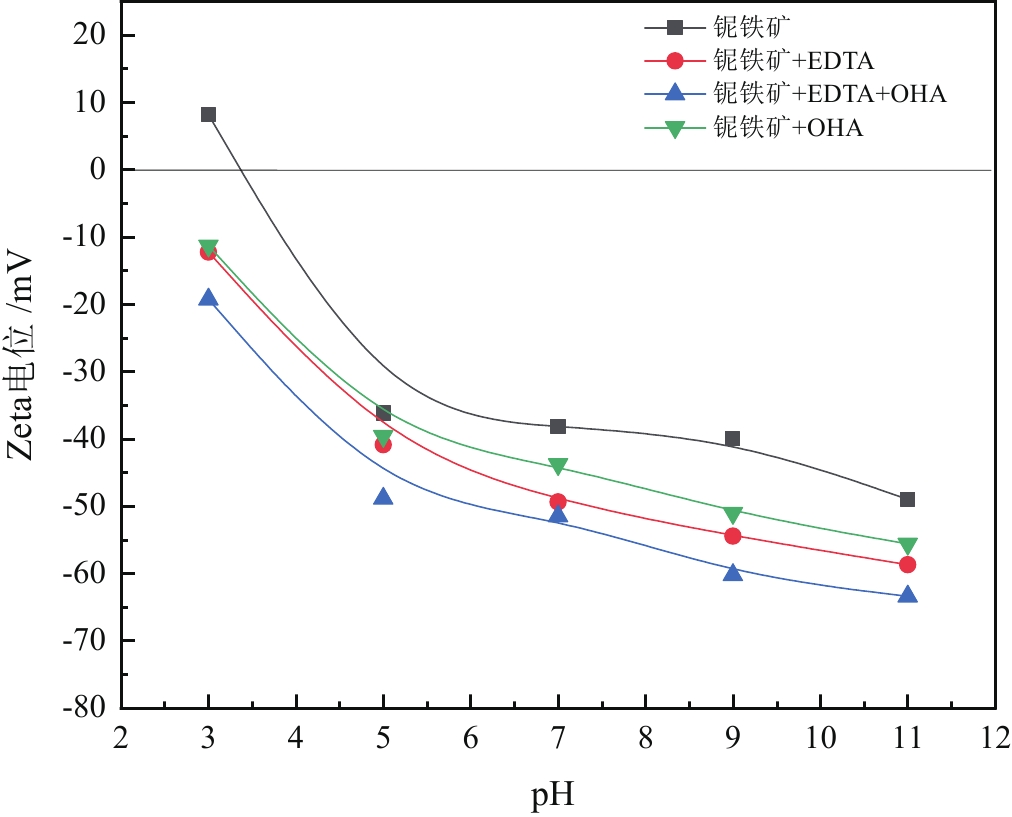
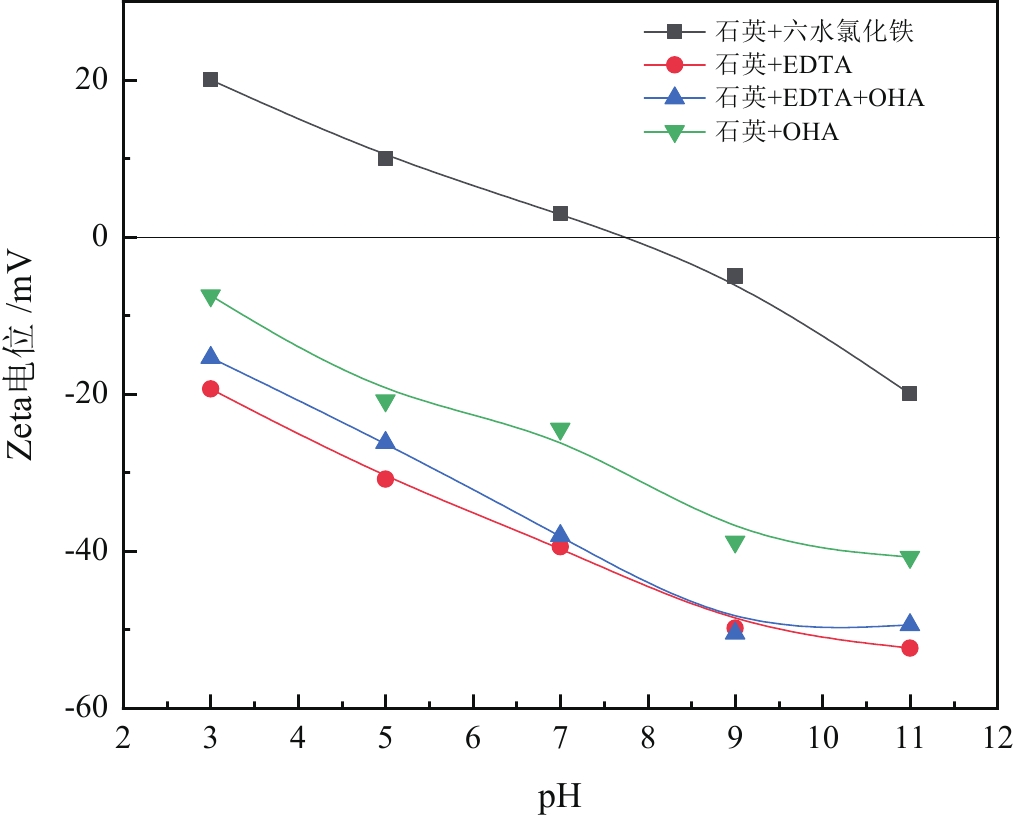
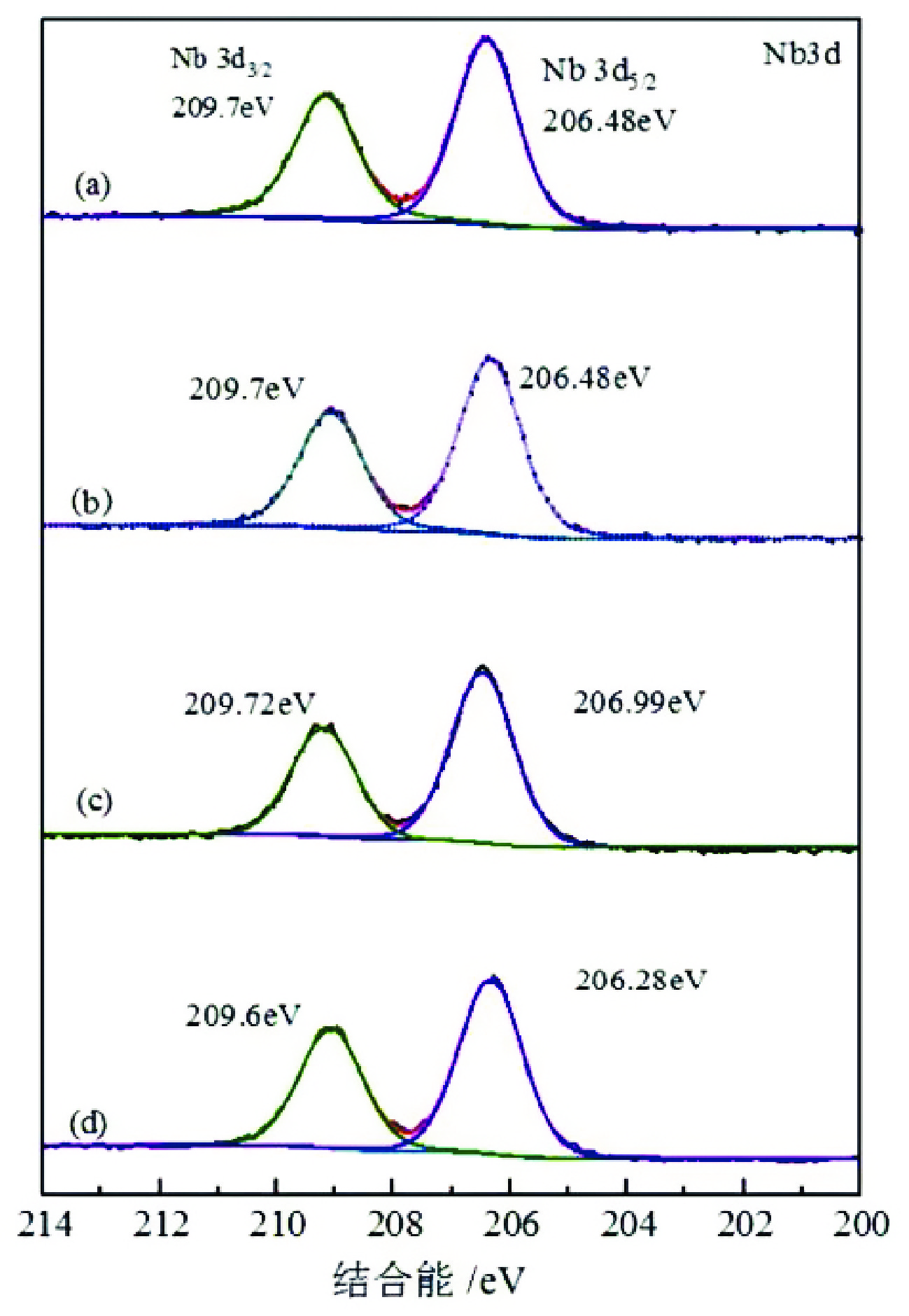
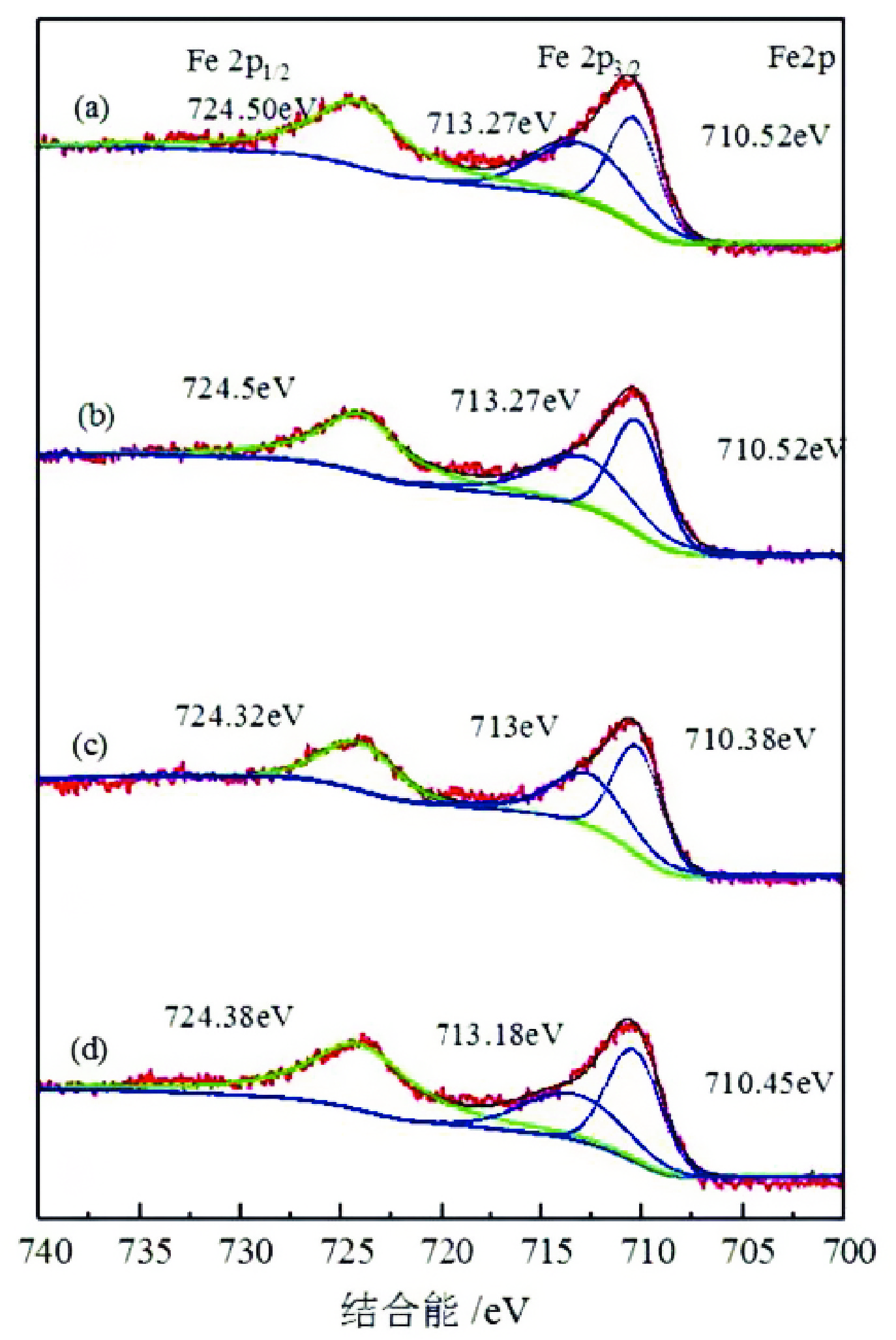
由于石英容易被金属阳离子活化,导致羟肟酸捕收剂浮选体系下铌矿物和脉石矿物石英的可浮性差异减小,增加了有用矿物和石英之间的分选难度。采用EDTA作为铌铁矿浮选中的石英抑制剂,通过单矿物试验、人工混合矿浮选试验、Zeta电位测试、接触角测试以及X射线光电子能谱仪检测等研究了铌铁矿以及石英的浮选行为和表面性质。当使用辛基羟肟酸(OHA)作为浮选捕收剂时,EDTA对活化后石英有较强的选择性抑制作用,因为EDTA对石英表面金属离子的络合溶解作用减少了OHA在石英表面的吸附,从而实现了铌铁矿和石英的有效分选。浮选试验结果表明,针对铌铁矿和石英质量比为1∶1的人工混合矿,在FeCl3·6H2O浓度为20 mg/L、EDTA用量为0.2 mmol/L、矿浆pH值为9.0、OHA浓度为0.05 mmol/L的条件下,可较好地实现铌铁矿和石英的浮选分离,铌铁矿精矿中Nb2O5的品位为56.84%,Nb2O5的回收率为72.54%,石英的品位为13.17%,石英的回收率为12.83%。
Abstract:As quartz is easily activated by metal cations, the floatability difference between niobium mineral and gangue mineral quartz decreases under hydroxamic acid collector flotation system, which increases the difficulty of separation between useful minerals and quartz. In this paper, EDTA was used as an inhibitor in the separation of niobium mineral from activated quartz. The flotation behavior and surface properties of niobite and quartz exposed to EDTA were studied by single mineral experiments, artificial mineral flotation experiments, Zeta potential tests, contact Angle tests and XPS photoelectron spectroscopy. When octyl hydroxamic acid (OHA) was used as flotation collector, EDTA as an inhibitor had a strong selective inhibition on activated quartz, because the complexation dissolution of EDTA on quartz surface reduced the adsorption of OHA on quartz surface. Flotation experiments results showed that the flotation separation of niobite from quartz was achieved at pH 9.0, FeCl3·6H2O dosage of 0.2 mmol/L, EDTA dosage of 0.2 mmol/L , and OHA dosage of 0.05 mmol/L. The recovery of Nb2O5 in niobite concentrate was 72.54%, and the grade of Nb2O5 was 56.84%. The recovery of SiO2 in niobite concentrate was 12.83%, and the grade of SiO2 was 13.17%.
-
Key words:
- niobite /
- quartz /
- EDTA /
- flotation separation /
- inhibitor /
- inhibition mechanism
-

-
表 1 单矿物化学成分分析结果
Table 1. Chemical analysis of pure minerals
/% 矿物 Nb2O5 TFe SiO2 MgO CaO 纯度 铌铁矿 75.65 16.38 3.21 − − 95.91 石英 − − 99.05 0.15 0.05 99.05 -
[1] 黎洁, 谢贤, 吕晋芳, 等. 铌矿资源概述及选矿技术研究进展[J]. 金属矿山, 2021(2): 120−126.
LI J, XIE X, LV J F, et al. Overview of niobium resources and research progress of beneficiation technology[J]. Metal Mine, 2021(2): 120−126.
[2] 苏立. 白云鄂博西矿铌富集机制及成矿相关性研究[D]. 北京: 中国矿业大学(北京), 2018.
SU L. Study on niobium enrichment mechanism and metallogenic correlation of Bayan obo xi ore deposit [D]. Beijing: China University of mining and Technology (Beijing), 2018.
[3] 郑文怡. 福建南平西坑钽铌矿稀有金属资源的综合利用分析[J]. 桂林理工大学学报, 2016, 36(1): 107−112.
ZHENG W Y. Comprehensive utilization analysis of rare metal resources in Xikeng tantalum niobium mine in Nanping of Fujian[J]. Journal of Guilin University of technology, 2016, 36(1): 107−112.
[4] 王鑫, 何东升, 刘爽, 等. 铌钽矿选矿研究进展[J]. 现代矿业, 2020, 36(4): 98−101.
WANG X, HE D S, LIU S, et al. Research progress of niobium tantalum ore dressing[J]. Modern Mining, 2020, 36(4): 98−101.
[5] 吴卫国, 孙传尧, 朱永楷. 有机螯合剂对活化石英的抑制及其作用机理[J]. 金属矿山, 2007(2): 33−37.
WU W G, SUN C Y, ZHU Y K. Inhibition of organic chelating agents on activated quartz and its mechanism[J]. Metal Mine, 2007(2): 33−37.
[6] 牛艳萍, 李亚, 许洪峰, 等. 油酸钠浮选体系中硅线石与石英的分离机理[J]. 有色金属(选矿部分), 2021(1): 122−127.
NIU Y P, LI Y, XU H F, et al. Separation mechanism of sillimanite and quartz in sodium oleate flotation system[J]. Mineral Processing Section, 2021(1): 122−127.
[7] 王介良, 曹钊, 王建英, 等. 辛基羟肟酸在氟碳铈矿表面的吸附机理[J]. 中南大学学报(自然科学版), 2019, 50(4): 762−770.
WANG J L, CAO Z, WANG J Y, et al. Adsorption mechanism of octyl-hydroxamic acid on bastnaesite[J]. Science and Technology, 2019, 50(4): 762−770.
[8] TIAN J, XU L H, SUN W, et al. The selective flotation separation of celestite from fluorite and calcite using a novel depressant EDTA[J]. Powder Technology, 2019, 352: 62−71. doi: 10.1016/j.powtec.2019.04.051
[9] LIU M X, LI H, JIANG T, et al. Flotation of coarse and fine pyrochlore using octyl hydroxamic acid and sodium oleate[J]. Minerals Engineering, 2019, 132: 191−201. doi: 10.1016/j.mineng.2018.12.014
[10] Y. MAKIMIZU, J. YOO, M. POORNAJAR, et al. Effects of low oxygen annealing on the photoelectrochemical water splitting properties of α-Fe2O3[J]. Journal of Materials Chemistry A: 2020, 8(3): 1315-1325.
-




 下载:
下载: