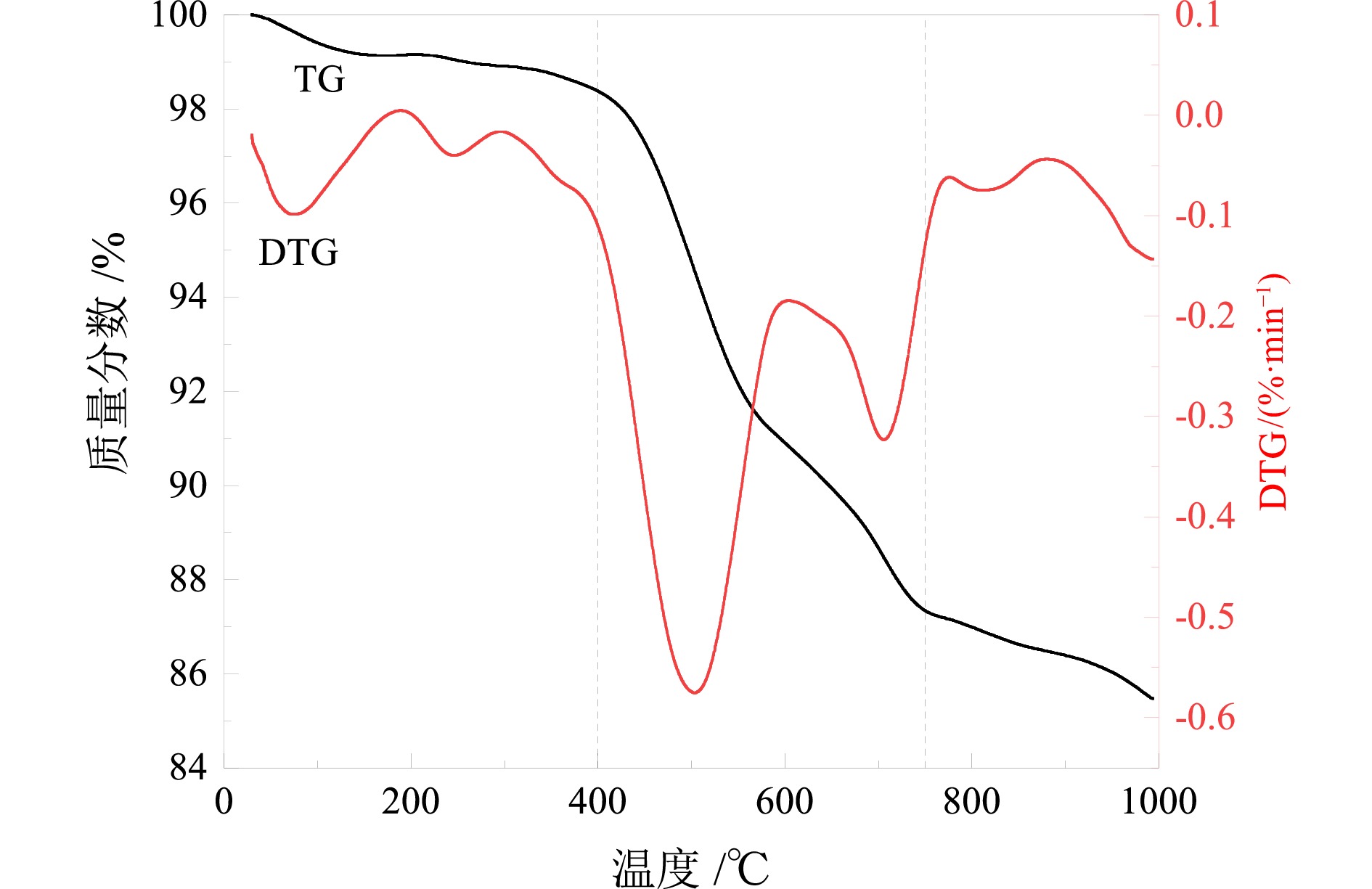
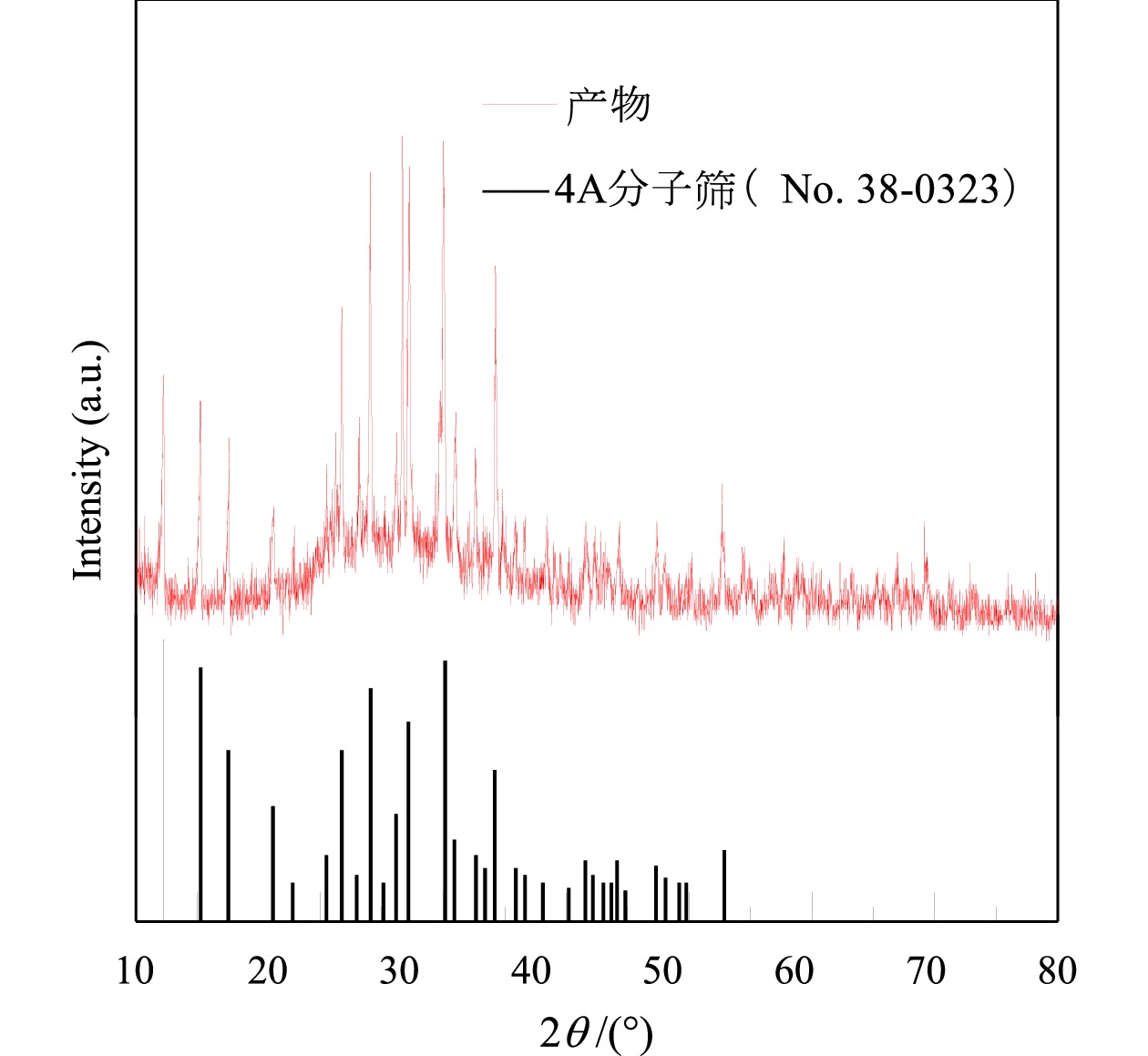
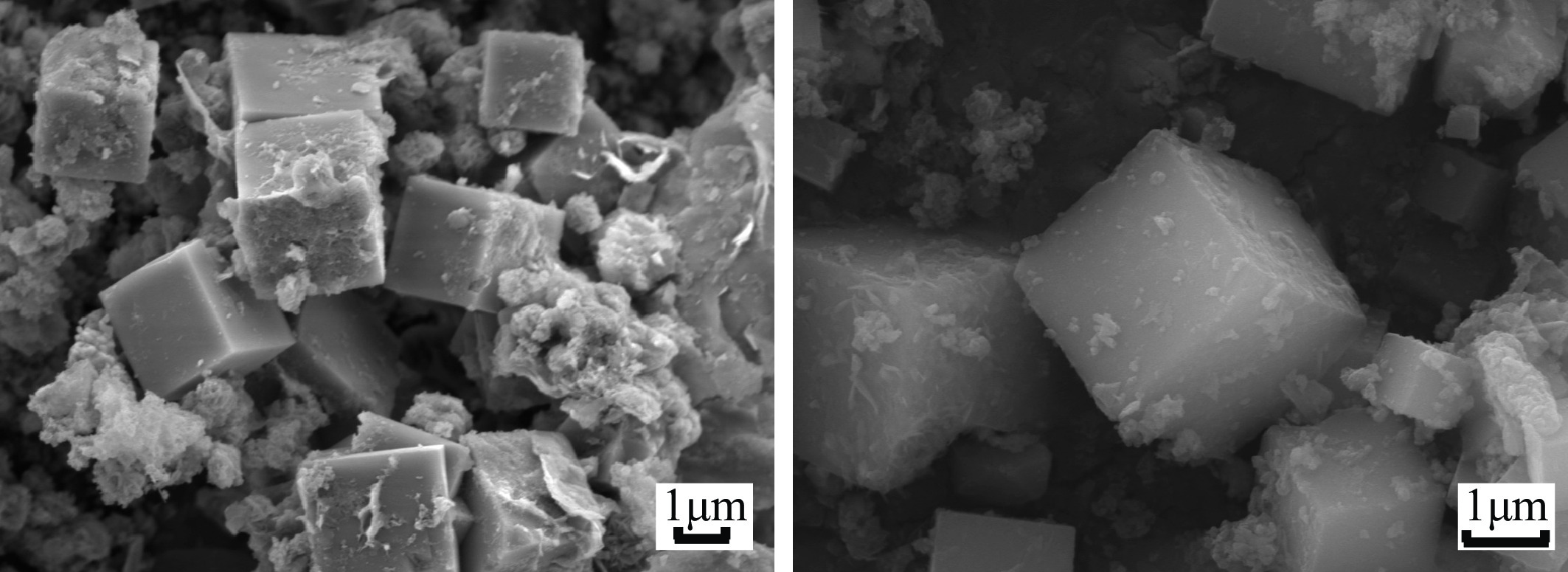
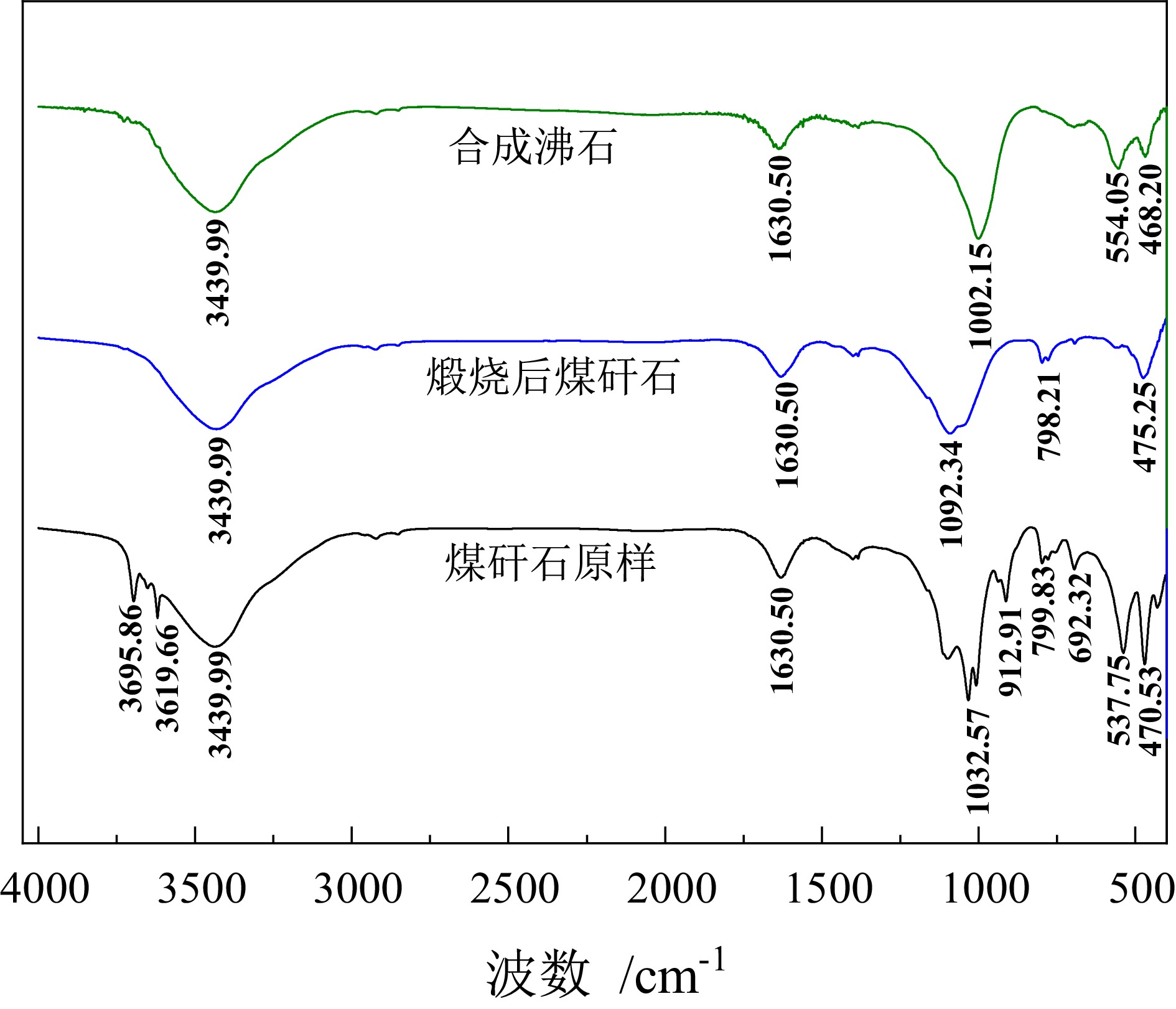
Preparation of Molecular Sieve from Coal Gangue and Adsorption Performance for Cu2+ in Acid Wastewater
-
摘要:
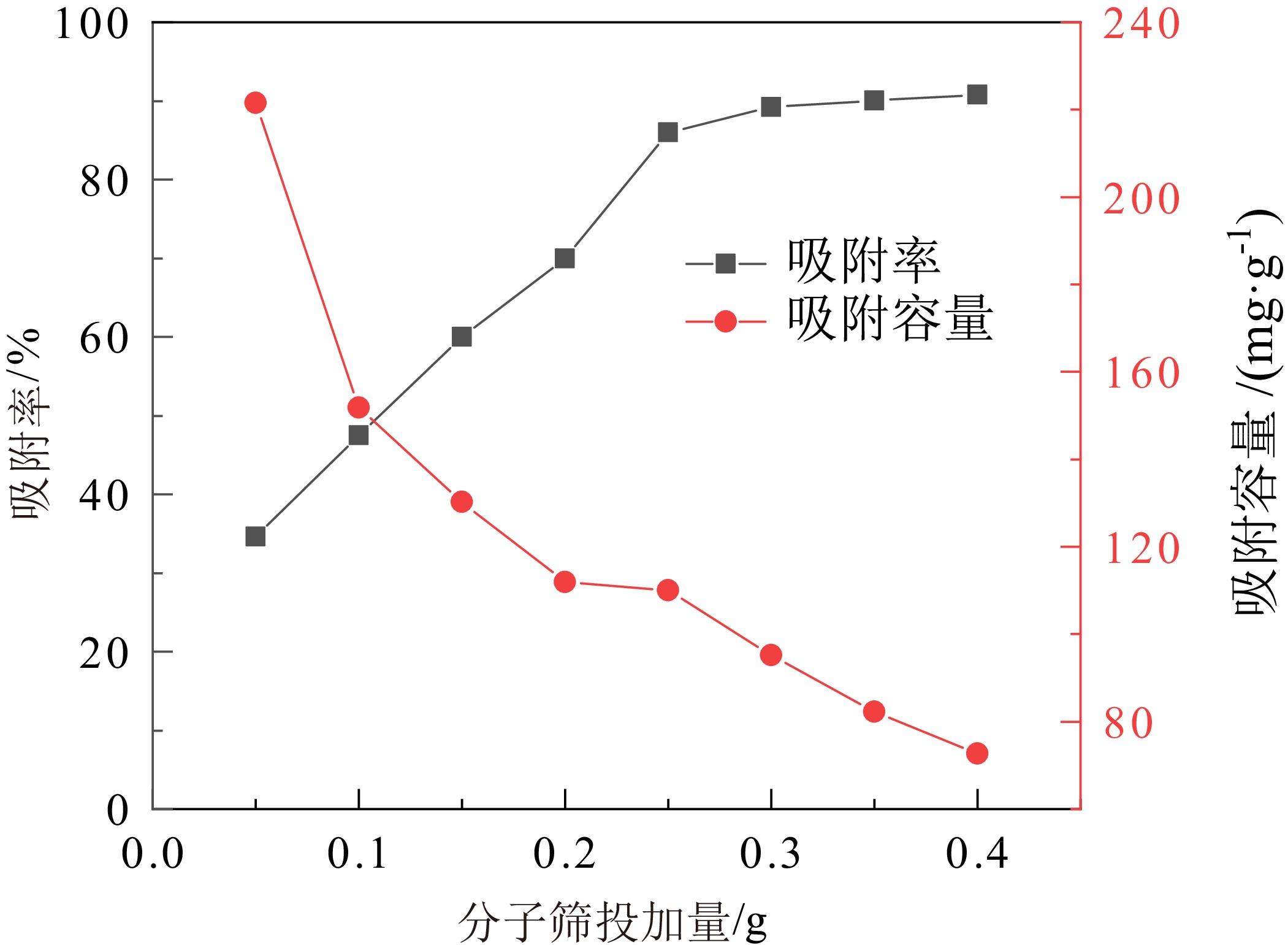
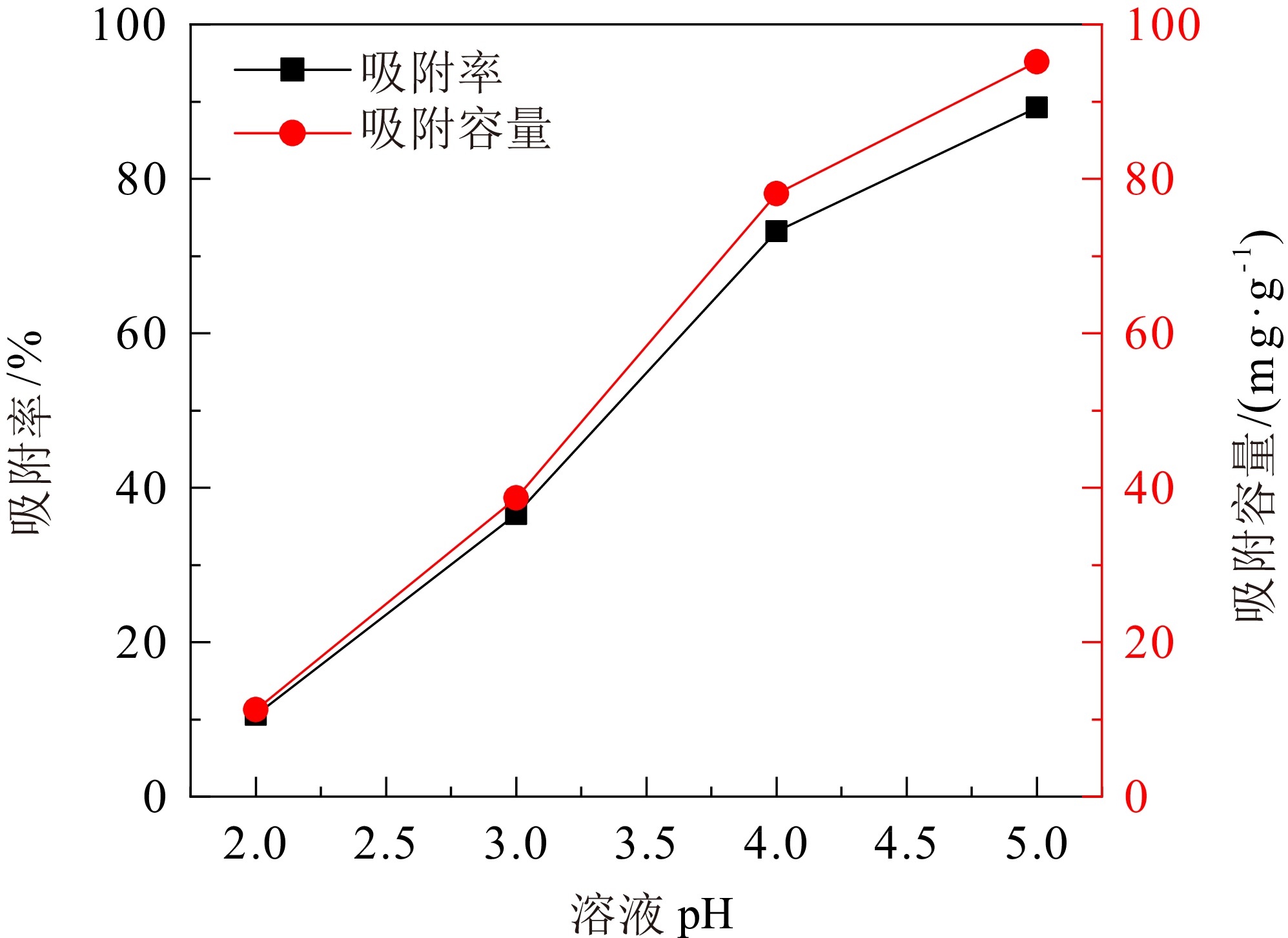
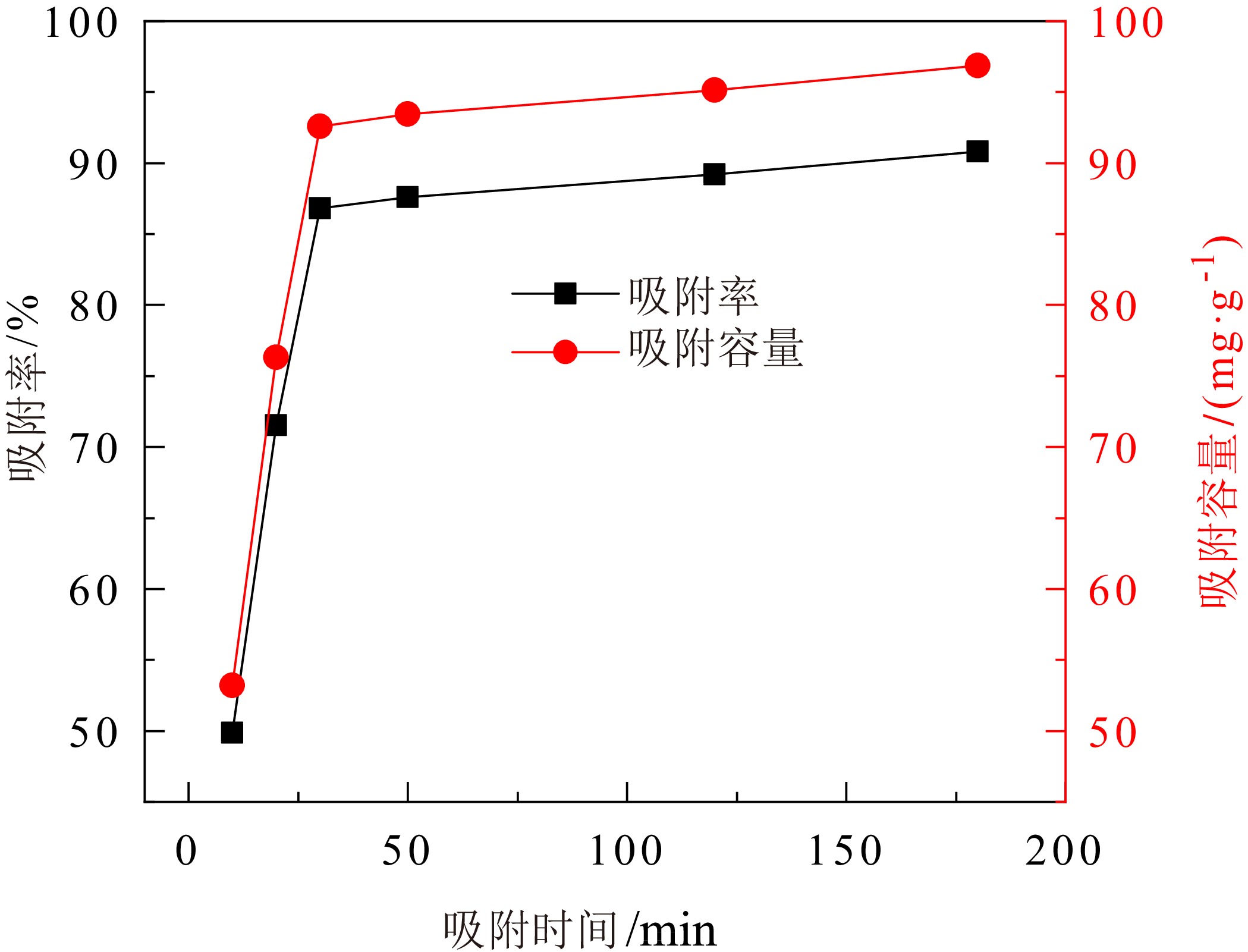
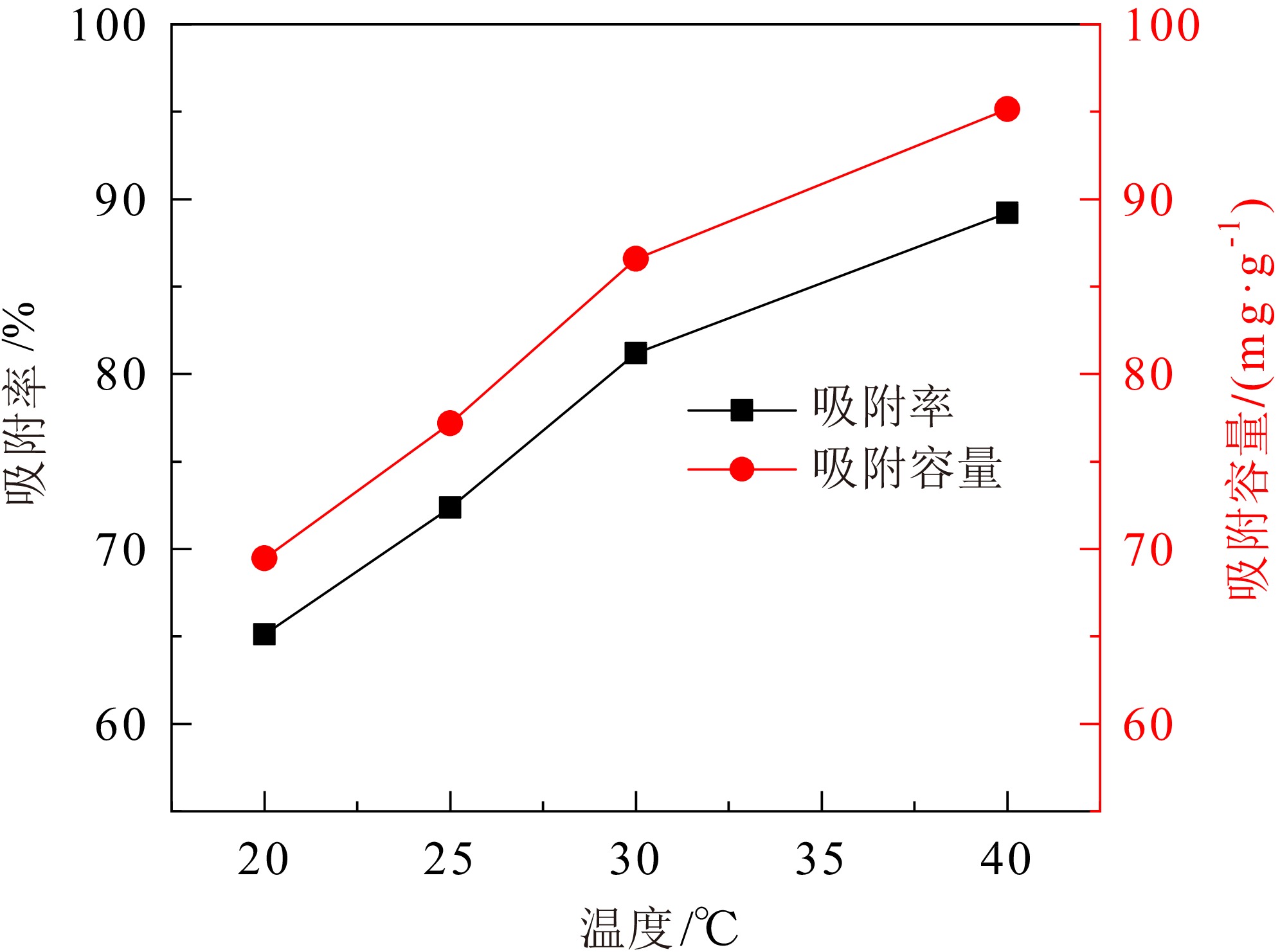
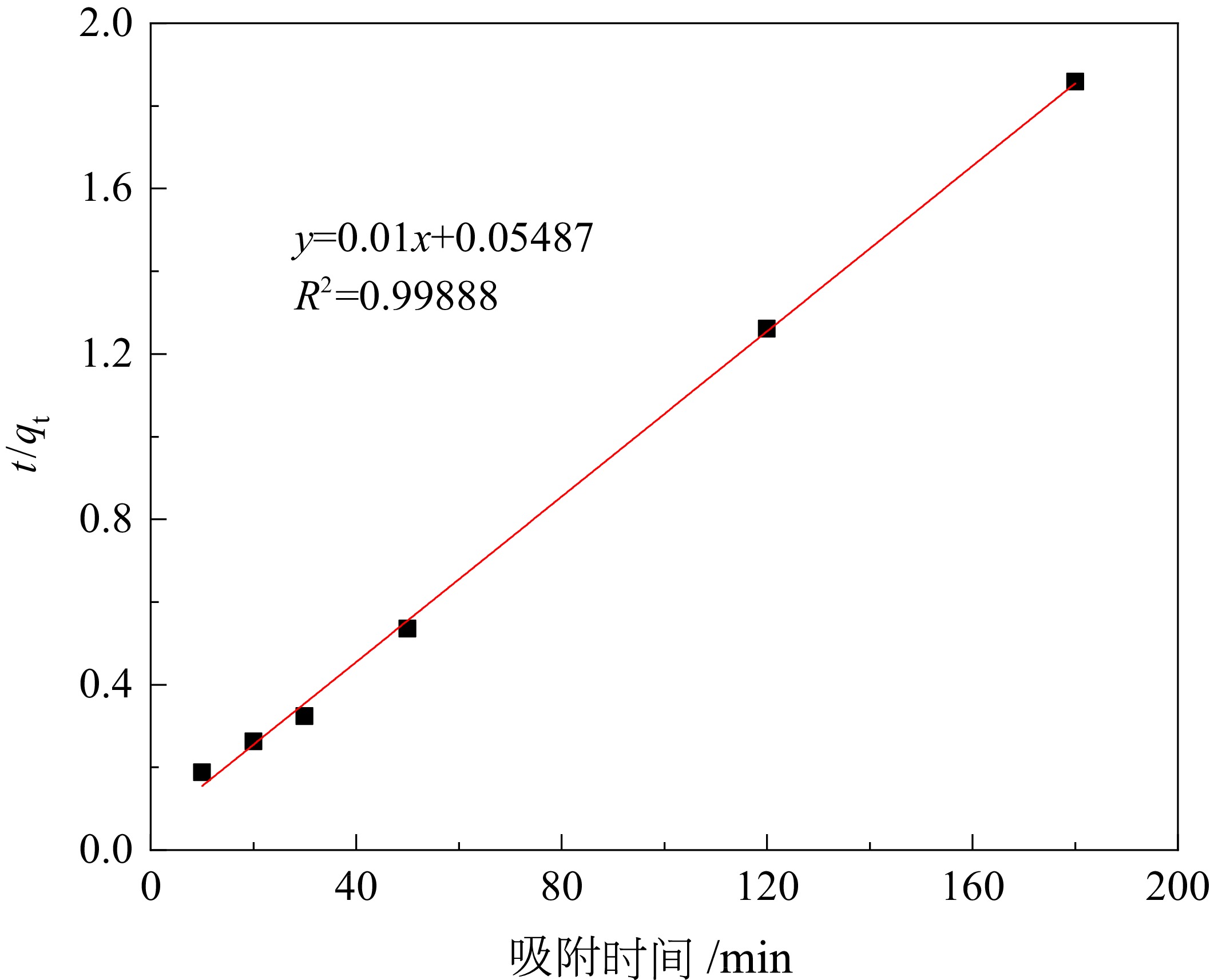
煤矸石中富含SiO2和Al2O3,可作为制备沸石分子筛的原料。对枣庄矿业集团煤矸石进行低温氧化、酸浸除杂和高温煅烧,再加以碱熔二次活化等预处理后,进行水热合成反应,制备了沸石分子筛。通过X射线衍射(XRD)、扫描电镜(SEM)、傅里叶红外光谱(FT−IR)对产物晶体结构、微观形貌和骨架结构进行了表征,表明产物为形态较佳且结晶度较高的4A分子筛。将所制备分子筛用于水中Cu2+的吸附,考察了分子筛用量、溶液pH值、吸附时间和温度对吸附效果的影响。结果表明在分子筛用量为6.0 g/L、pH值为5.0、吸附时间为180 min、吸附温度为40 ℃时,对Cu2+浓度为 0.01 mol/L的酸性废水中Cu2+的吸附率可达89.2%。吸附动力学分析表明吸附过程符合准二级动力学方程,化学吸附在吸附过程中起主导作用。
Abstract:Coal gangue is rich in SiO2 and Al2O3, and can be used as raw material for zeolite molecular sieve. A molecular sieve was prepared by coal gangue from Zaozhuang Mining Group, through the process of low−temperature oxidation, acid leaching, high−temperature calcination, activated by alkali melting, and further hydrothermal reaction. The crystal structure, morphology, and skeleton structure of the product were characterized by X−ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FT−IR) respectively. The results showed that the product was a 4A molecular sieve with good morphology and high crystallinity. The prepared molecular sieve was used for the adsorption of Cu2+ in aqueous solution, and the effects of molecular sieve dosage, solution pH value, adsorption time, and temperature on the adsorption effect were investigated. Adsorption experiments showed that its adsorption rate for Cu2+ reached 89.2% in the acid wastewater containing 0.01 mol/L of copper, when the dosage was 6.0 g/L, the pH value was 5.0, the adsorption time was 180 min, and the adsorption temperature was 40 ℃. The adsorption kinetics analysis showed that the adsorption process conformed to quasi second−order kinetic equation, and chemical adsorption played a dominant role in the adsorption process.
-
Key words:
- coal gangue /
- activation /
- hydrothermal synthesis /
- molecular sieve /
- adsorption
-

-
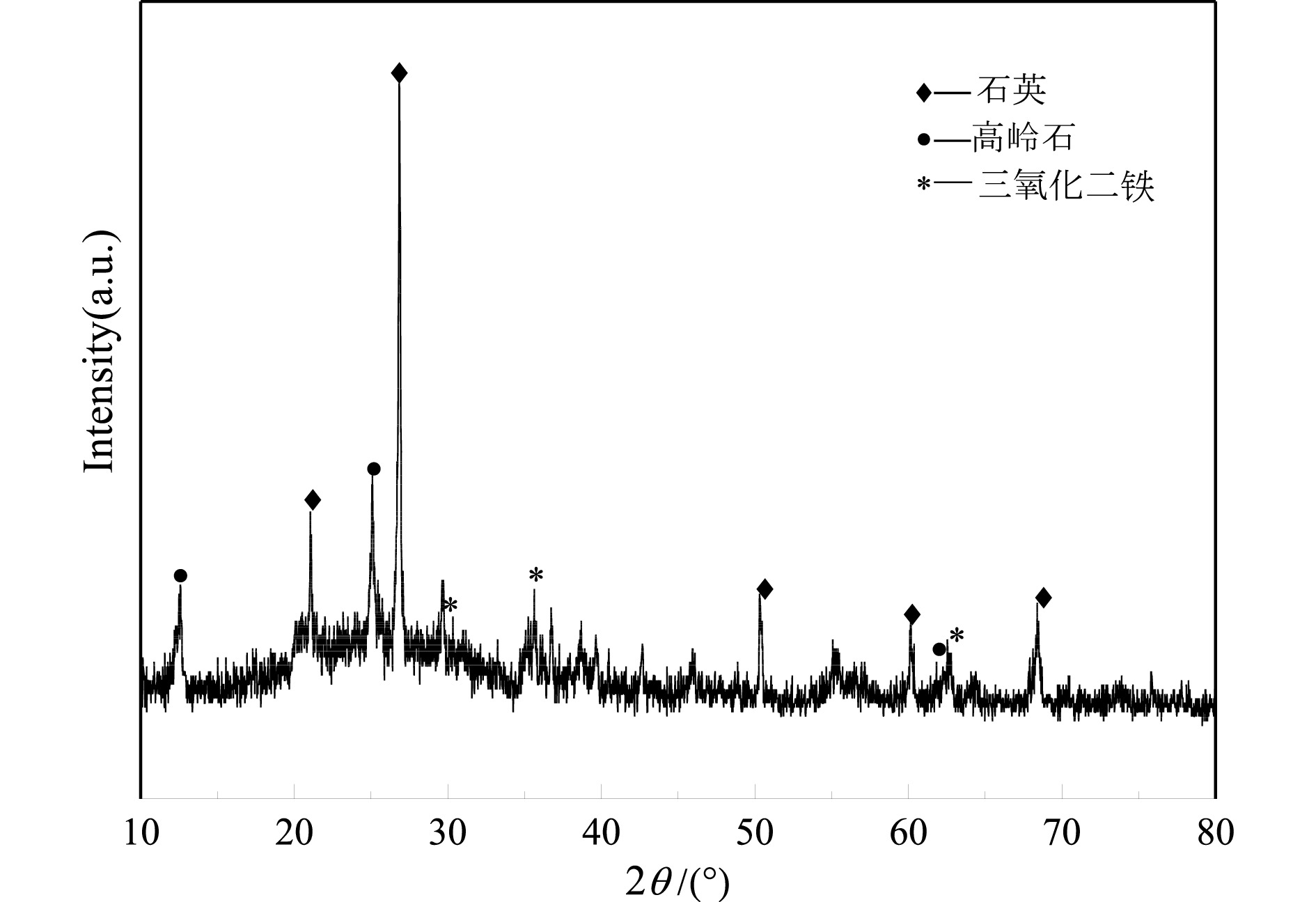
表 1 煤矸石原料化学多元素分析结果
Table 1. Multi−elements analysis results of coal gangue
/% 成分 SiO2 Al2O3 Fe2O3 CaO TiO2 其他 含量 36.57 7.70 35.40 9.27 2.33 8.73 -
[1] 郭振坤, 范雯阳, 周珊, 等. 利用煤矸石制备4A分子筛及吸附性能的研究[J]. 无机盐工业, 2017, 49(2): 78−81.
GUO Z K, FAN W Y, ZHOU S, et al. Preparation and adsorption properties of 4A zeolite from coal gangue[J]. Inorganic Chemicals Industry, 2017, 49(2): 78−81.
[2] 吴涛, 杜美利, 司玉成, 等. 黄陵煤矸石制备4A分子筛的研究[J]. 硅酸盐通报, 2015, 34(5): 1348−1353.
WU T, DU M L, SI Y C, et al. Preparation of 4A molecular sieve using Huangling coal gangue[J]. Bulletin of the Chinese Ceramic Society, 2015, 34(5): 1348−1353.
[3] LI J Y, WANG J M. Comprehensive utilization and environmental risks of coal gangue: A review[J]. Journal of Cleaner Production, 2019, 239: 117946. doi: 10.1016/j.jclepro.2019.117946
[4] 周楠, 姚依南, 宋卫剑, 等. 煤矿矸石处理技术现状与展望[J]. 采矿与安全工程学报, 2020, 37(1): 136−146.
ZHOU N, YAO Y N, SONG W J, et al. Present situation and prospect of coal gangue treatment technology[J]. Journal of Mining & Safety Engineering, 2020, 37(1): 136−146.
[5] 李振, 雪佳, 朱张磊, 等. 煤矸石综合利用研究进展[J]. 矿产保护与利用, 2021, 41(6): 165−178.
LI Z, XUE J, ZHU Z L, et al. Research progress on comprehensive utilization of coal gangue[J]. Conservation and Utilization of Mineral Resources, 2021, 41(6): 165−178.
[6] 贾鲁涛, 吴倩云. 煤矸石特性及其资源化综合利用现状[J]. 煤炭技术, 2019, 38(11): 37−40.
JIA L T, WU Q Y. Properties and comprehensive utilization status of coal gangue resource[J]. Coal Technology, 2019, 38(11): 37−40.
[7] 郭丽, 李平, 田红丽, 等. 高硅煤矸石一步碱熔法合成4A分子筛研究[J]. 应用化工, 2016, 45(9): 1726−1728.
GUO L, LI P, TIAN H L, et al. Synthesis of 4A−zeolite from coal gangue with high silica of Ningdong mining by one−step alkali fusion[J]. Applied Chemical Industry, 2016, 45(9): 1726−1728.
[8] 孔德顺, 吴红, 连明磊. 高铁含量煤矸石制备4A分子筛的研究[J]. 硅酸盐通报, 2019, 38(9): 2999−3003.
KONG D S, WU H, LIAN M L. Study on the preparation of 4A zeolite from coal gangue with high iron content[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(9): 2999−3003.
[9] 许红亮, 程维高, 李牧, 等. 煤矸石制备4A分子筛工艺研究[J]. 非金属矿, 2011, 34(2): 14−16.
XU H L, CHENG W G, LI M, et al. Preparation of 4A−zeolite from coal gangue[J]. Non−Metallic Mines, 2011, 34(2): 14−16.
[10] 孔德顺, 连明磊, 范佳鑫, 等. 劣质煤矸石合成4A沸石分子筛[J]. 中国陶瓷, 2013, 49(6): 37−39.
KONG D S, LIAN M L, FAN J X, et al. Synthesis of 4A molecular sieves from poor quality coal gangue[J]. China Ceramics, 2013, 49(6): 37−39.
[11] 孔德顺, 李琳, 范佳鑫, 等. 高铁高硅煤矸石制备P型分子筛[J]. 硅酸盐通报, 2013, 32(6): 1052−1056.
KONG D S, LI L, FAN J X, et al. Preparation of P type molecular sieves from gangue of high iron and high silica content[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(6): 1052−1056.
[12] 杨建利, 杜美利, 于春侠, 等. 煤矸石制备4A分子筛的研究[J]. 西安科技大学学报, 2013, 33(1): 61−65. doi: 10.3969/j.issn.1672-9315.2013.01.012
YANG J L, DU M L, YU C X, et al. Preparation of molecular sieve using coal gangue[J]. Journal of Xi´ an University of Science and Technology, 2013, 33(1): 61−65. doi: 10.3969/j.issn.1672-9315.2013.01.012
[13] 任根宽. 用煤矸石合成4A沸石分子筛处理氨氮废水[J]. 环境工程学报, 2014, 8(4): 1533−1538.
REN G K. Removal of ammonia−nitrogen in wastewater with 4A zeolite molecular sieve synthesized from coal gangue[J]. Chinese Journal of Environmental Engineering, 2014, 8(4): 1533−1538.
[14] CHEN J L, LU X W. Equilibrium and kinetics studies of Cd (II) sorption on zeolite NaX synthesized from coal gangue[J]. Journal of Water Reuse and Desalination, 2018, 8(1): 94−101. doi: 10.2166/wrd.2016.137
[15] BU N J, LIU X M, SONG S L, et al. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution[J]. Advanced Powder Technology, 2020, 31: 2699−2710. doi: 10.1016/j.apt.2020.04.035
[16] 陈建龙, 卢新卫, 张萌萌, 等. 矸石基X 型分子筛对水中Co2+、Cu2+、Cd2+和 Cr3+的去除[J]. 环境工程学报, 2017, 8(9): 3625−3632.
CHEN J L, LU X W, ZHANG M M, et al. Removal of Co2+, Cu2+, Cd2+ and Cr3+ from aqueous solution by zeolite X prepared from coal gangue[J]. Chinese Journal of Environmental Engineering, 2017, 8(9): 3625−3632.
[17] 万琼, 孙永庆, 张新艳, 等. 煤矸石基沸石分子筛在水处理中的研究进展[J]. 水处理技术, 2021, 47(9): 1−5.
WAN Q, SUN Y Q, ZHANG X Y, et al. Research progress of coal gangue−based zeolite molecular sieve in water treatment[J]. Technology of Water Treatment, 2021, 47(9): 1−5.
[18] GE Q L, MOEEN M, TIAN Q, et al. Highly effective removal of Pb2+ in aqueous solution by Na−X zeolite derived from coal gangue[J]. Environmental Science and Pollution Research, 2020, 27(7): 7398−7408. doi: 10.1007/s11356-019-07412-z
[19] 梁止水, 高琦, 刘豪伟, 等. 煤矸石制备 NaX型分子筛及其对Cd2+的吸附性能[J]. 东南大学学报(自然科学版), 2020, 50(4): 741−747. doi: 10.3969/j.issn.1001-0505.2020.04.019
LIANG Z S, GAO Q, LIU H W, et al. Synthesis of NaX zeolite from coal gangue and its adsorption capability for Cd2+[J]. Journal of Southeast University (Natural Science Edition), 2020, 50(4): 741−747. doi: 10.3969/j.issn.1001-0505.2020.04.019
[20] 夏彬, 王晓丽, 张艳. 鄂尔多斯煤矸石合成 A 型沸石吸附剂试验研究[J]. 硅酸盐通报, 2018, 37(4): 1462−1466.
XIA B, WANG X L, ZHANG Y. Synthesis of zeolite A adsorbents by coal gangue in Erdos[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(4): 1462−1466.
[21] 马志军, 郑云生, 周智静, 等. 煤矸石基方沸石分子筛对Cr3+的吸附性能研究[J]. 非金属矿, 2022, 45(1): 78−82. doi: 10.3969/j.issn.1000-8098.2022.01.021
MA Z J, ZHENG Y S, ZHOU Z J, et al. Study on the adsorption of Cr3+ on coal gangue based analcite molecular sieve[J]. Non−metallic Mines, 2022, 45(1): 78−82. doi: 10.3969/j.issn.1000-8098.2022.01.021
[22] 康超, 乔金鹏, 杨胜超, 等. 煤矸石中有价关键金属活化提取研究进展[J]. 化工学报, 2023, 74(7): 2783−2799.
KANG C, QIAO J P, YANG S C, et al. Research progress on activation extraction of valuable metals in coal gangue[J]. CIESC Journal, 2023, 74(7): 2783−2799.
[23] CAO Z, CAO Y D, DONG H J, et al. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue[J]. International Journal of Mineral Processing, 2016, 146: 23−28. doi: 10.1016/j.minpro.2015.11.008
[24] 胡芳华, 王万绪, 杨效益, 等. 煤系高岭土合成洗涤剂助剂用纯4A沸石[J]. 煤炭学报, 2009, 34(10): 1364−1369. doi: 10.3321/j.issn:0253-9993.2009.10.013
HU F H, WANG W X, YANG X Y, et al. Pure zeolite 4A synthesized from coal kaolinite for use as detergents builder[J]. Journal of China Coal Society, 2009, 34(10): 1364−1369. doi: 10.3321/j.issn:0253-9993.2009.10.013
[25] KONG D S, JIANG R L. Preparation of NaA zeolite from high iron and quartz contents coal gangue by acid leaching−alkali melting activation and hydrothermal synthesis[J]. Crystals, 2021, 11(10): 1198. doi: 10.3390/cryst11101198
[26] 漆才文, 尹艳山, 吴紫华, 等. 湘西煤燃烧过程中矿物质演化规律的FTIR, XRD和XPS研究[J]. 煤炭转化, 2022, 45(3): 18−25.
QI C W, YIN Y S, WU Z H, et al. Study on mineral transformation during the combustion of Xiangxi coal by FTIR, XRD and XPS[J]. Coal Conversion, 2022, 45(3): 18−25.
-



 下载:
下载: