Review on Fine Mineral Flotation: Increasing Apparent Particle Size and Decreasing Bubble Diameter
-
摘要:
微细粒矿物的浮选回收是世界性难题,增大颗粒表观直径与减小气泡尺寸为解决该难题的有效途径。论文综述了增大颗粒表观直径的四种方法:疏水絮凝浮选、载体浮选、选择性絮凝浮选和剪切絮凝浮选,详细阐述了其在矿物加工领域中的应用及机理,尤其是增大颗粒表观粒径过程中新药剂的最新研究进展及应用领域。从减小气泡尺寸角度出发,以微纳米气泡在矿物加工领域的应用研究为落脚点,阐述了微纳米气泡现有的稳定性机理,为后续微纳米气泡稳定性机理的深入研究提供参考;系统介绍了微纳米气泡在不同种类微细粒矿物浮选中的应用现状;从微纳米气泡与颗粒间界面作用机理出发,详细阐述了微纳米气泡在界面作用中的角色;举例介绍了微纳米气泡浮选设备的研究进展。提出微纳米气泡强化细粒浮选的机理需要进一步明确,基于微纳米气泡、矿浆精准可控的微纳米气泡浮选设备是微细粒矿物浮选的重要研究方向。
Abstract:Fine mineral flotation is a worldwide problem, and increasing the apparent particle diameter and reducing the bubble size are effective ways to solve it. In this paper, four methods of increasing the apparent particle diameter were reviewed: hydrophobic flocculation flotation, carrier flotation, selective flocculation flotation and shear flocculation flotation, and their application and mechanism in mineral processing were expounded in detail, especially the latest research progress and application fields of new reagents in the process of increasing the apparent particle size. From the perspective of reducing the bubble size, the stability mechanism of micro nano bubbles in the field of mineral processing was expounded, which provided a reference for its further research. The application of micro nano bubble in the flotation of different kinds of fine minerals is systematically introduced. Based on the mechanism of interfacial interaction between microbubbles and particles, the role of micro−nano bubbles in interfacial interaction was described in detail. Finally, the research progress of microbubble flotation equipment was introduced through cases. It was proposed that the mechanism of micro−bubble enhanced fine flotation needed to be further clarified, and micro−bubble flotation equipment based on precisely controlling of micro−bubble and pulp was an important research direction of micro−fine mineral flotation.
-
Key words:
- fine particle /
- micro−nano bubble /
- flotation /
- flocculation /
- flotation column /
- interface interaction
-

-
图 1 微细颗粒的流线运动[3]
Figure 1.
图 2 硫化矿物回收率随粒度变化[5]
Figure 2.
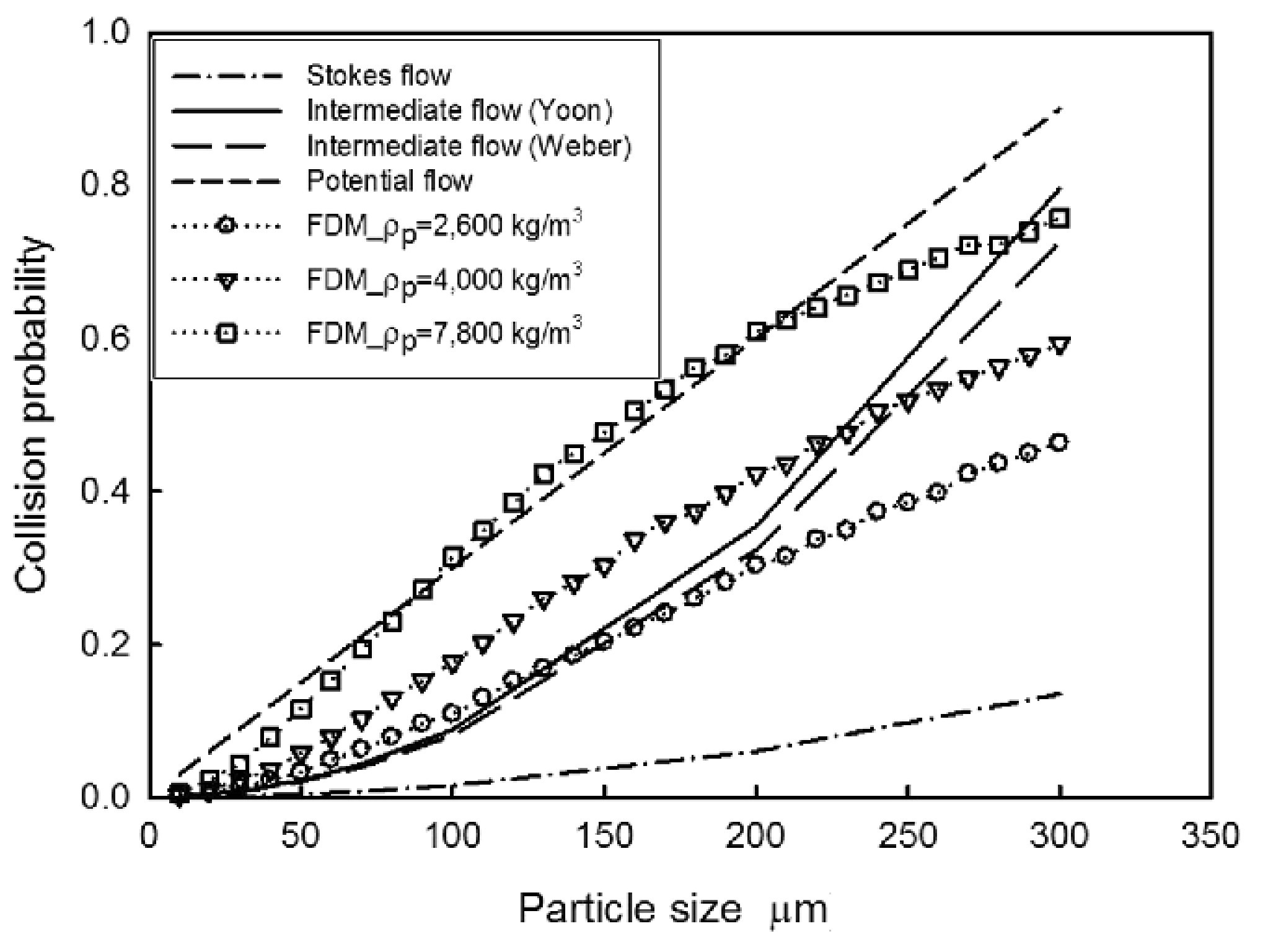
图 3 不同颗粒密度的碰撞概率与粒径的关系[6]
Figure 3.
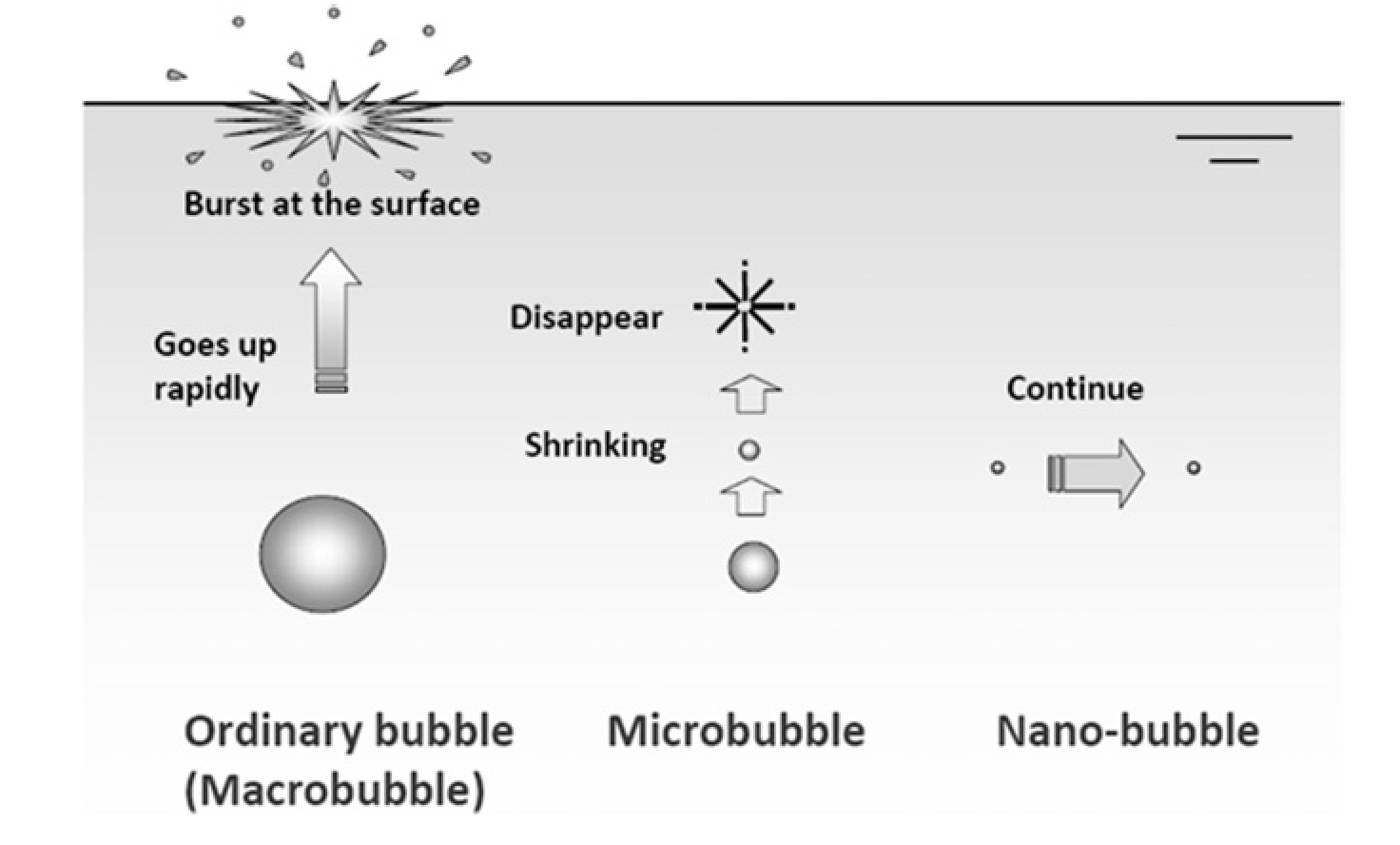
图 4 宏观、微米和纳米气泡的示意图[40]
Figure 4.
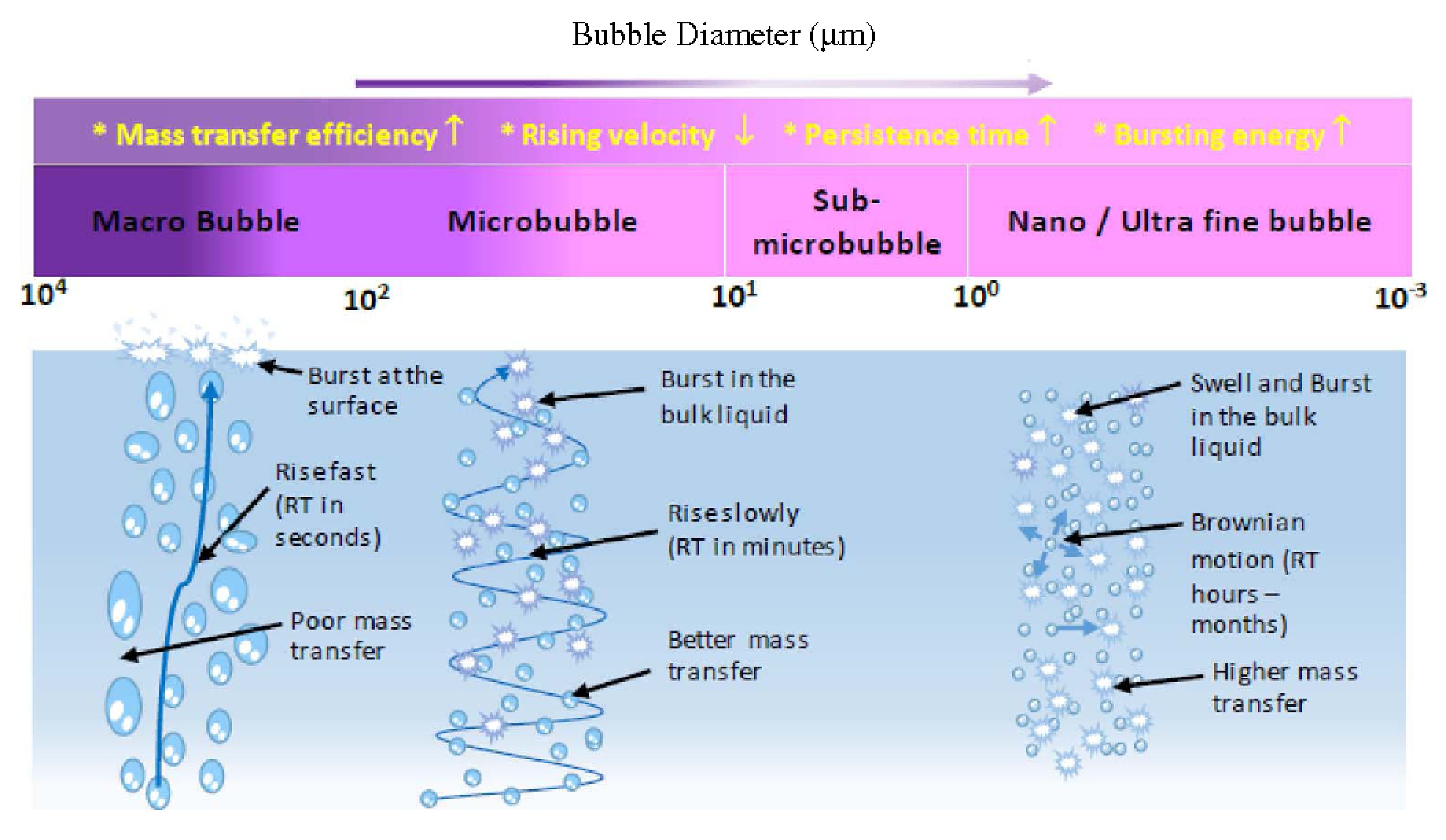
图 5 气泡尺寸和主要特性[41]
Figure 5.
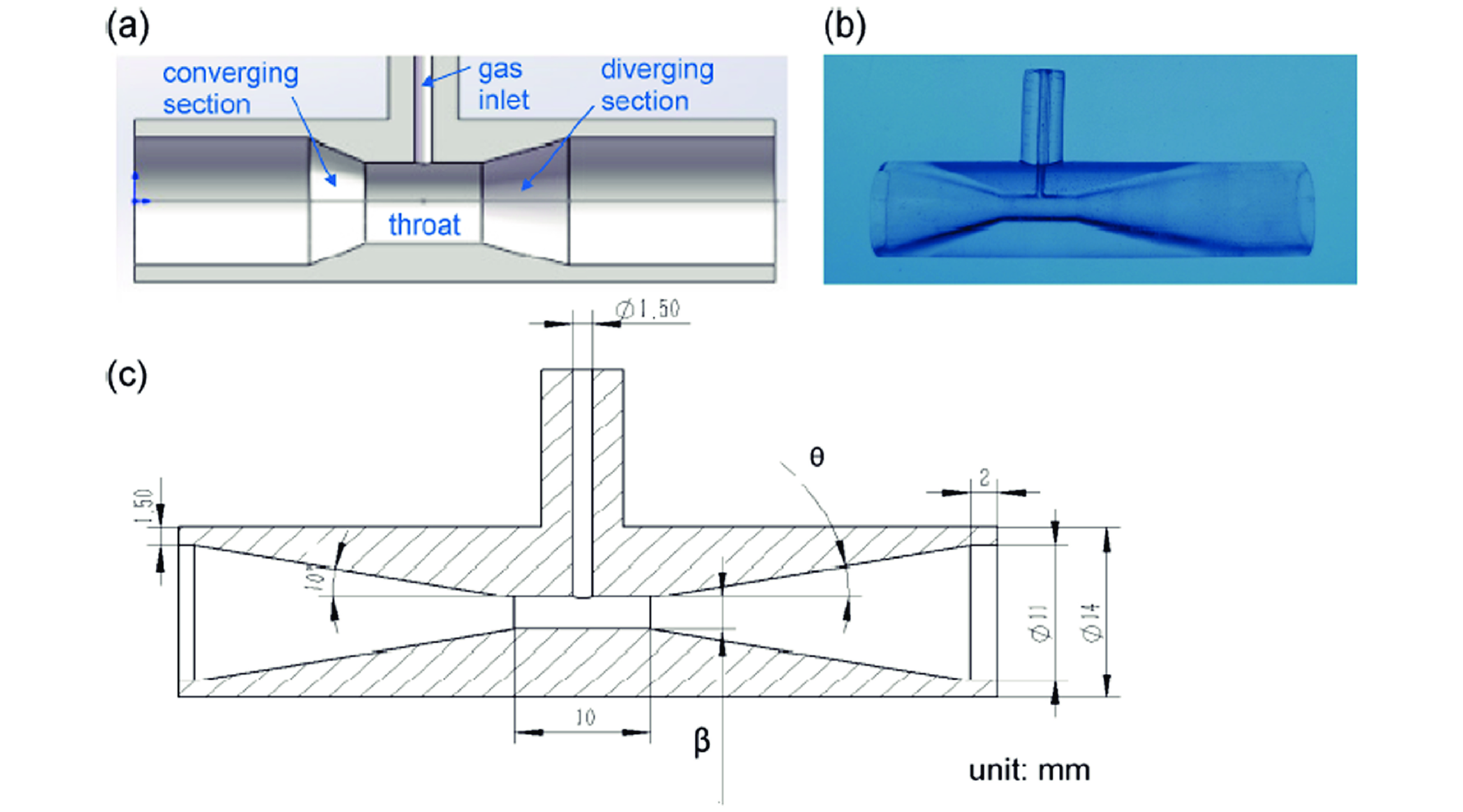
图 6 (a)文丘里气泡发生器示意图;(b)3D打印文丘里气泡发生器照片;(c)几何参数[43]
Figure 6.
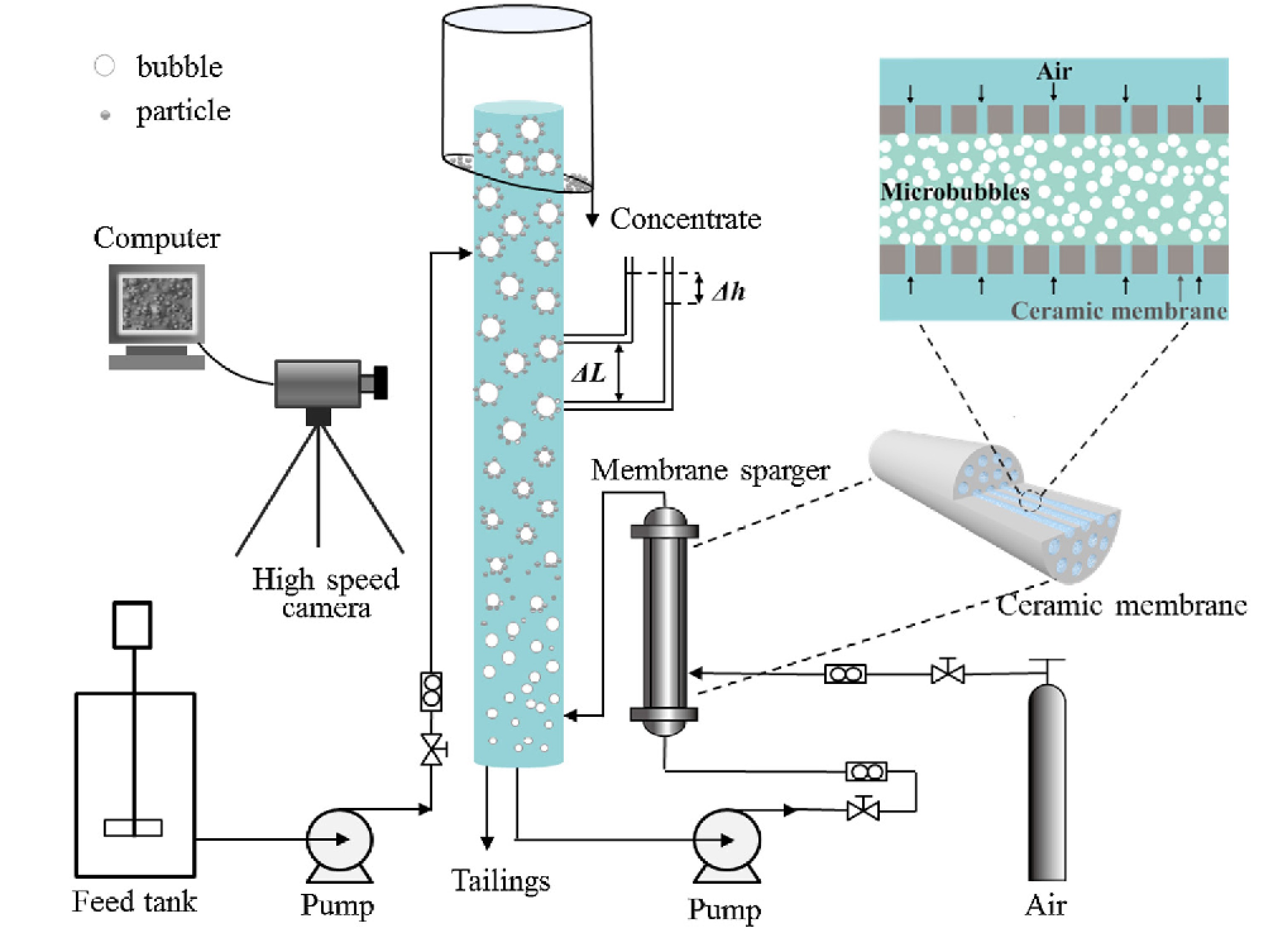
图 7 带膜分布器的微泡浮选柱示意图[44]
Figure 7.
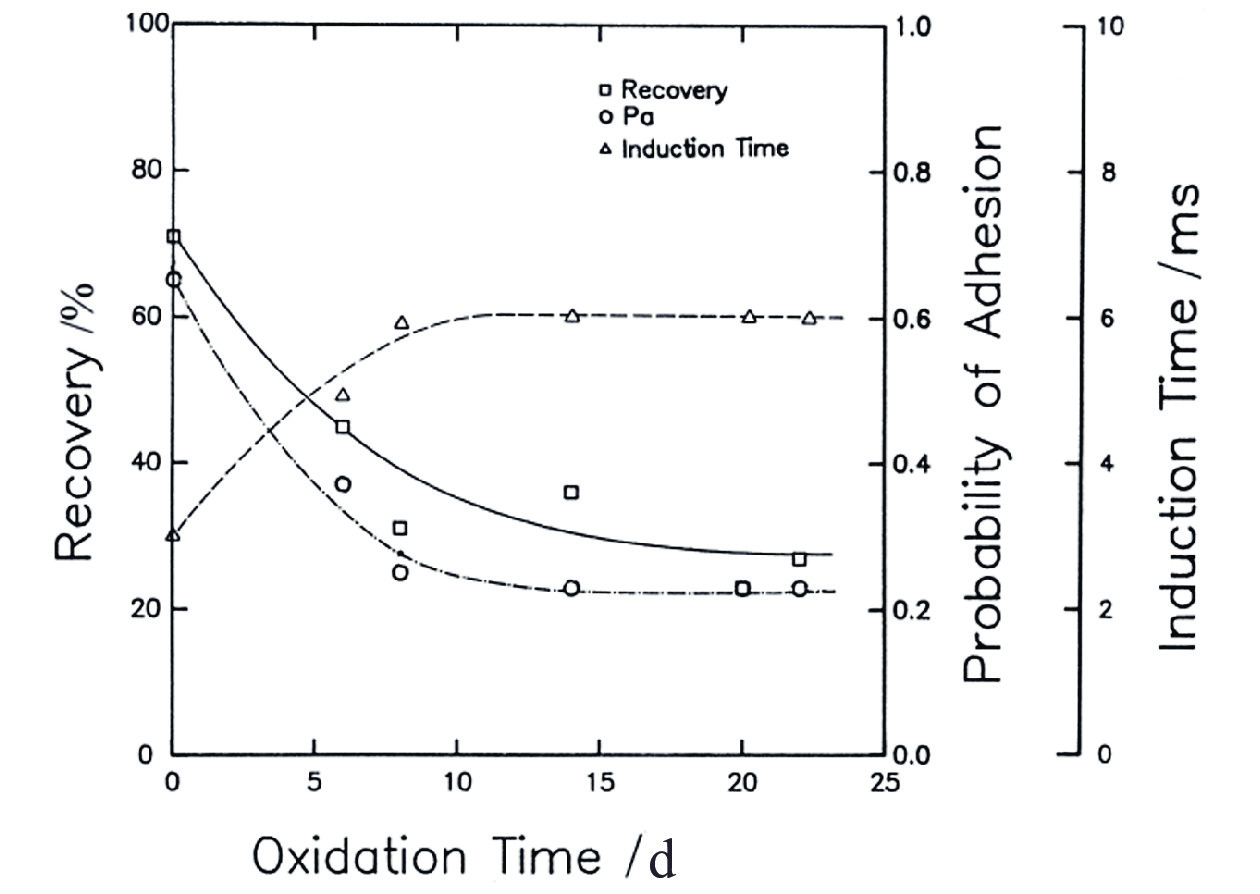
图 8 矿粒被气泡附着的概率与感应时间的关系[67]
Figure 8.
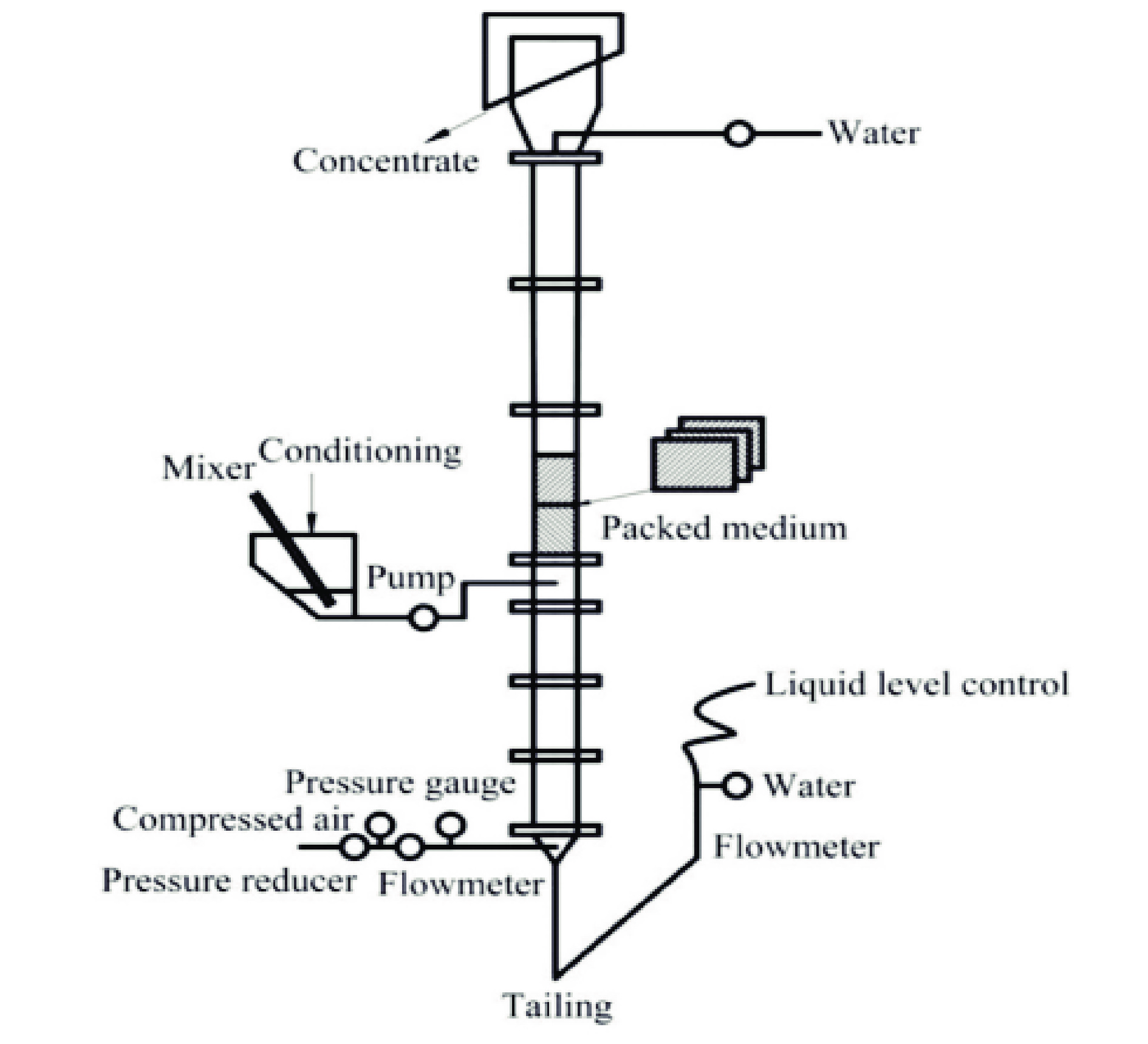
图 10 填充浮选塔示意图[82]
Figure 10.
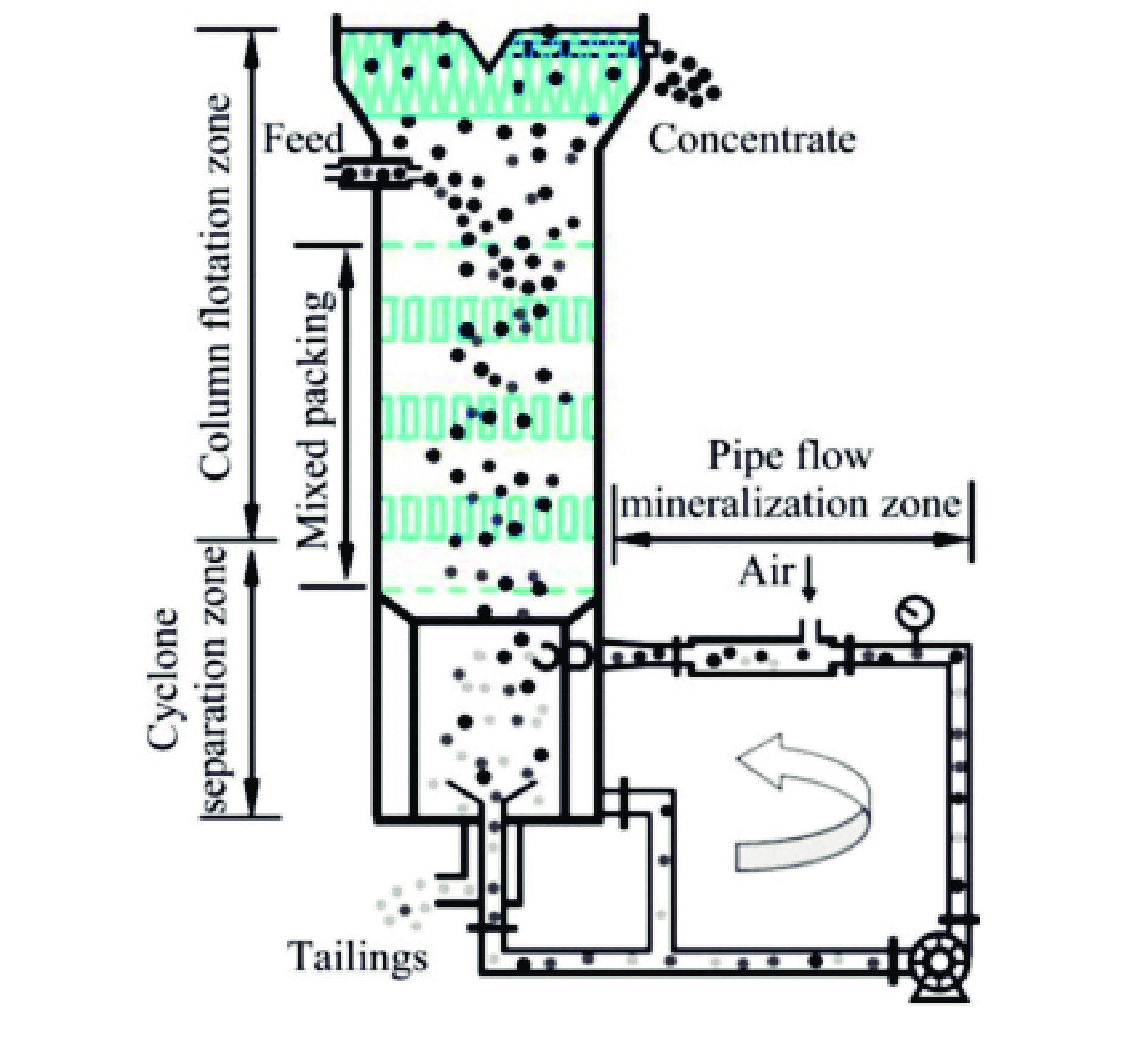
图 11 旋流静态微泡浮选柱示意图[82]
Figure 11.
表 1 常见的温度敏感型絮凝剂 [29]
Table 1. Summary of mineral systems for common temperature−responsive flocculants[29]
缩写 聚合物名称 适用矿物 PAA 聚丙烯酸 二氧化钛、氧化铝、赤铁矿(铁矿石)、高岭石 PAEMA 聚(2−氨基乙基甲基丙烯酰胺盐酸盐) 高岭石、油砂 PAM 聚丙烯酰胺 油砂 PAOPA 聚(3−丙烯酰氧基丙酸) 方解石 PBAAM 聚(N−叔丁基丙烯酰胺) 高岭石 PDADMAC 聚(二烯丙基二甲基氯化铵) 二氧化钛、方解石、油砂 PDMAPAA 聚(N,N−二甲氨基丙基丙烯酰胺) 二氧化钛、煤、高岭石 PDMAPMA 聚(N,N−二甲氨基丙基甲基丙烯酰胺) 煤、黏土(高岭土、蒙脱石、石英) PDQA 聚(二甲氨基丙烯酸乙酯氯化四酯) 氧化铝、石英 PMAAB 聚(5−甲基丙烯酰胺基−1,2−苯并硼唑) 高岭石 PNIPAM 聚(N−异丙基丙烯酰胺) 氧化铝、方解石、石英、二氧化钛、
黏土(高岭石、蒙脱石)、石英、煤、黄铜矿与石英混合物、
赤铁矿(铁矿石)、高岭石、油砂PNVCL 聚(N−乙烯基己内酰胺) 高岭石、石英 PTBA 聚(丙烯酸叔丁酯) 氧化铝 甲基纤维素 锆石 -
[1] WANG X, YUAN S, LIU J, et al. Nanobubble−enhanced flotation of ultrafine molybdenite and the associated mechanism[J]. Journal of Molecular Liquids, 2022: 118312.
[2] CHITALU C, CLAYTON B. The role of the water–air and pulp−froth interfaces on particle detachment: Impact of particle size, type, contact angle and bubble rise velocity[J]. Minerals Engineering, 2024, 205: 108453.
[3] REN L, ZHANG Z, ZENG W, et al. Adhesion between nanobubbles and fine cassiterite particles[J]. International Journal of Mining Science and Technology, 2023, 33: 503−509.
[4] WANG D, LIU Q. Hydrodynamics of froth flotation and its effects on fine and ultrafine mineral particle flotation: A literature review[J]. Minerals Engineering, 2021, 173: 107220.
[5] FENG D, ALDRICH C. Effect of particle size on flotation performance of complex sulphide ores[J]. Minerals Engineering, 1999, 12: 721−731.
[6] JE J, LEE D, KWON J, et al. Simulation of bubble−particle collision process and estimation of collision probability using a coupled smoothed particle hydrodynamics−discrete element method model[J]. Minerals Engineering, 2022, 176: 107309.
[7] SHAHBAZI B, REZAI B, JAVAD K. The effect of hydrodynamic parameters on probability of bubble–particle collision and attachment[J]. Minerals Engineering, 2009, 22: 57−63. doi: 10.1016/j.mineng.2008.03.013
[8] REN H, CHEN W, ZHENG Y, et al. Effect of hydrophobic group on flocculation properties and dewatering efficiency of cationic acrylamide copolymers[J]. Reactive and Functional Polymers, 2007, 67(7): 601−608. doi: 10.1016/j.reactfunctpolym.2007.03.008
[9] WANG Z, LIU N, ZOU D. Interface adsorption mechanism of the improved flotation of fine pyrite by hydrophobic flocculation[J]. Separation and Purification Technology, 2021, 275: 119245. doi: 10.1016/j.seppur.2021.119245
[10] HUANG X, XIAO W, ZHAO H, et al. Hydrophobic flocculation flotation of rutile fines in presence of styryl phosphonic acid[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(7): 1424−1432. doi: 10.1016/S1003-6326(18)64781-8
[11] YIN W, YANG X, ZHOU D, et al. Shear hydrophobic flocculation and flotation of ultrafine Anshan hematite using sodium oleate[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 652−664. doi: 10.1016/S1003-6326(11)60762-0
[12] ZHOU S, WANG X, BU X, et al. A novel flotation technique combining carrier flotation and cavitation bubbles to enhance separation efficiency of ultra−fine particles[J]. Ultrasonics Sonochemistry, 2020, 64: 105005. doi: 10.1016/j.ultsonch.2020.105005
[13] WANG W, HUANG Y, MA H, et al. Enhanced phosphate pollutant removal from liquid via adsorption flotation strategy: Synergic effect of Fe (Ⅲ)−fulvic acid carrier and CTAB[J]. Separation and Purification Technology, 2023, 326: 124791. doi: 10.1016/j.seppur.2023.124791
[14] SUBRAHMANYAM T. V, ERIC K. S, FORSSBERG. Fine particles processing: shear−flocculation and carrier flotation — a review[J]. International Journal of Mineral Processing, 1990, 30(3/4): 265−286. doi: 10.1016/0301-7516(90)90019-U
[15] LI D, YIN W, LIU Q, et al. Interactions between fine and coarse hematite particles in aqueous suspension and their implications for flotation[J]. Minerals Engineering, 2017, 114: 74−81. doi: 10.1016/j.mineng.2017.09.012
[16] MUHAMMAD B, MAYUMI I, RIKU A, et al. Heterogenous carrier flotation technique for recovering finely ground chalcopyrite particles using coarse pyrite particles as a carrier[J]. Minerals Engineering, 2022, 180: 107518. doi: 10.1016/j.mineng.2022.107518
[17] MUHAMMAD B, MAYUMI I, KANAMI K, et al. Effects of coarse chalcopyrite on flotation behavior of fine chalcopyrite[J]. Minerals Engineering, 2021, 163: 106776. doi: 10.1016/j.mineng.2021.106776
[18] LUVER E, DARWIN E, PEDRO G, et al. The depressing effect of an anionic polyacrylamide on molybdenite flotation and the importance of polymer anionicity[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 629: 127506. doi: 10.1016/j.colsurfa.2021.127506
[19] ELIZAVETA F. Shear selective and temperature responsive flocculation: A comparison of fine particle flotation techniques[J]. International Journal of Mineral Processing, 2011, 99(1/2/3/4): 1−10. doi: 10.1016/j.minpro.2011.02.001
[20] CHENG K, WU X Q, TANG H H, et al. The flotation of fine hematite by selective flocculation using sodium polyacrylate[J]. Minerals Engineering, 2022(176): 107273.
[21] ZOU W, GONG L, HUANG J, et al. Adsorption of hydrophobically modified polyacrylamide P(AM−NaAA−C16DMAAC) on model coal and clay surfaces and the effect on selective flocculation of fine coal[J]. Minerals Engineering, 2019, 142: 105887. doi: 10.1016/j.mineng.2019.105887
[22] REN H, LI Y, ZHANG S, et al. Flocculation of kaolin suspension with the adsorption of N, N−disubstituted hydrophobically modified polyacrylamide[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2008, 317(1-3): 388−393. doi: 10.1016/j.colsurfa.2007.11.007
[23] KHAI E L, NORHASHIMAH M, BENG T P, et al. Comparative study on the effectiveness of hydrophobically modified cationic polyacrylamide groups in the flocculation of kaolin[J]. Desalination, 2011, 270(1/2/3): 206−213. doi: 10.1016/j.desal.2010.11.047
[24] MA J, SHI J, DING H, et al. Synthesis of cationic polyacrylamide by low−pressure UV initiation for turbidity water flocculation[J]. Chemical Engineering Journal, 2017, 312: 20−29. doi: 10.1016/j.cej.2016.11.114
[25] LV S, PENG W, CAO Y, et al. Synthesis and characterisation of a novel pH−sensitive flocculant and its flocculation performance[J]. Journal of Molecular Liquids, 2022, 348: 118480. doi: 10.1016/j.molliq.2022.118480
[26] PENG W, LV S, CAO Y, et al. A novel pH−responsive flocculant for efficient separation and recovery of Cu and Mo from secondary resources via selective flocculation−flotation[J]. Journal of Cleaner Production, 2023, 395: 136463. doi: 10.1016/j.jclepro.2023.136463
[27] JOHN P, GREG G, GEORGE V. Temperature responsive flocculation and solid–liquid separations with charged random copolymers of poly(N−isopropyl acrylamide)[J]. Journal of Colloid and Interface Science. 2011, 360(1): 61−70
[28] GEORGE V, LI HH, JOHN P, et al. Temperature responsive polymers as multiple function reagents in mineral processing[J]. Advanced Powder Technology, 2009, 203: 273−279.
[29] WEI S, LUKE A, ELIZAVETA F, et al. A review of temperature−responsive polymers as novel reagents for solid−liquid separation and froth flotation of minerals[J]. Minerals Engineering, 2018, 123: 144−159. doi: 10.1016/j.mineng.2018.03.027
[30] LEONARD J. Shear−flocculation of ultrafine scheelite in sodium oleate solutions[J]. Journal of Colloid and Interface Science, 1975, 50(2): 307−318. doi: 10.1016/0021-9797(75)90234-9
[31] NI C, ZHANG Q, JIN M, et al. Effect of high−speed shear flocculation on the flotation kinetics of ultrafine microcrystalline graphite[J]. Powder Technology, 2022, 396((Part A)): 345−353. doi: 10.1016/j.powtec.2021.10.041
[32] ALPER O, KIRAZ E. Use of ultrasonic treatment as a pre−phase in the shear flocculation process[J]. Ultrasonics, 2023, 134: 107052. doi: 10.1016/j.ultras.2023.107052
[33] PASCOE R, DOHERTY E. Shear flocculation and flotation of hematite using sodium oleate[J]. International Journal of Mineral Processing, 1997, 51(1/2/3/4): 269−282. doi: 10.1016/S0301-7516(97)00033-1
[34] BHASKAR R, SUBRAHMANYAM T V, SUN Z, et al. Shear−flocculation of quartz[J]. International Journal of Mineral Processing, 1991, 32(3/4): 283−294. doi: 10.1016/0301-7516(91)90074-S
[35] OZKAN A, UCBEYIAY H, AYDOGAN S. Shear flocculation of celestite with anionic surfactants and effects of some inorganic dispersants[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2006, 281(1/2/3): 92−98.
[36] 曹阳. 还原体系下解抑活化细粒锡石浮选的应用基础研究[D]. 昆明理工大学, 2023.
CAO Y. Applied basic research on the flotation of deactivated fine cassiterite in reduction system [D]. Kunming University of Science and Technology, 2023.
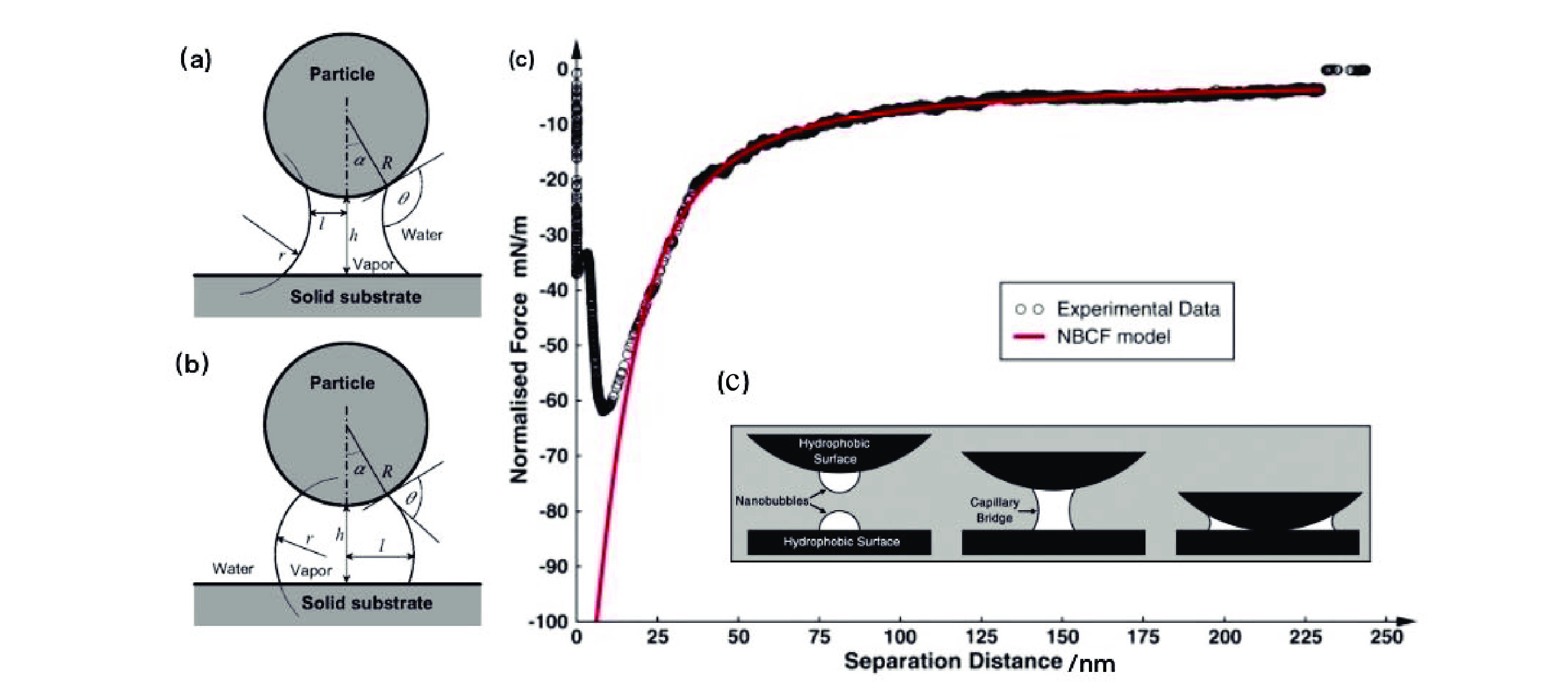
[37] XIE L, WANG J, LU Q, et al. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives[J]. Advances in Colloid and Interface Science, 2021, 295: 102491. doi: 10.1016/j.cis.2021.102491
[38] 任浏祎, 曾维能, 张喆怡, 等. 微纳米气泡对微细粒锡石团聚影响的可视化研究[J]. 中国有色金属学报, 2022, 32(5): 1479−1490.
REN L Y, ZENG W N, ZHANG Z Y, et al. Visualization of effect of micro−nano bubbles on agglomeration of fine cassiterite[J]. The Chinses Journal of Nonferrous Metals, 2022, 32(5): 1479−1490.
[39] HAN G, CHEN S, SU S, et al. A review and perspective on micro and nanobubbles: What they are and why they matter[J]. Minerals Engineering, 2022, 189: 107906. doi: 10.1016/j.mineng.2022.107906
[40] ASHUTOSH A, WUN J, LIU Y. Principle and applications of microbubble and nanobubble technology for water treatment[J]. Chemosphere, 2011, 84(9): 1175−1180. doi: 10.1016/j.chemosphere.2011.05.054
[41] TEMESGEN T, BUI T T, HAN M, et al. Micro and nanobubble technologies as a new horizon for water−treatment techniques: A review[J]. Advances in Colloid and Interface Science, 2017, 246: 40−51. doi: 10.1016/j.cis.2017.06.011
[42] JAMES J, MARK A. Lipid monolayer collapse and microbubble stability [J]. Advances in Colloid and Interface Science, 2012, 183–184: 82−99.
[43] RUAN J, ZHOU H, DING Z M, et al. Machine learning−aided characterization of microbubbles for venturi bubble generator[J]. Chemical Engineering Journal, 2023, 465: 142763. doi: 10.1016/j.cej.2023.142763
[44] TAO X, LIU Y, JIANG H, et al. Microbubble generation with shear flow on large−area membrane for fine particle flotation[J]. Chemical Engineering and Processing − Process Intensification, 2019, 145: 107671. doi: 10.1016/j.cep.2019.107671
[45] MIN U, YEO C, GHISLAIN B, et al. Industrial application of microbubble generation methods for recovering fine particles through froth flotation: A review of the state−of−the−art and perspectives[J]. Advances in Colloid and Interface Science, 2023, 322: 103047. doi: 10.1016/j.cis.2023.103047
[46] JIA J, ZHU Z, CHEN H, et al. Full life circle of micro−nano bubbles: Generation, characterization and applications[J]. Chemical Engineering Journal, 2023, 471: 144621. doi: 10.1016/j.cej.2023.144621
[47] ZAHRA T, EBRAHIM A, MAHDI S, et al. Nano−microbubbles and feed size interaction in lead and zinc sulfide minerals flotation[J]. Chemical Engineering and Processing − Process Intensification, 2023, 189: 109401. doi: 10.1016/j.cep.2023.109401
[48] ZHANG F, SUN L, YANG H, et al. Recent advances for understanding the role of nanobubbles in particles flotation[J]. Advances in Colloid and Interface Science, 2021, 291: 102403. doi: 10.1016/j.cis.2021.102403
[49] FENG W, NARESH S, SIMON S. Drainage mechanism of microbubble dispersion and factors influencing its stability[J]. Journal of Colloid and Interface Science, 2009, 337(2): 548−554. doi: 10.1016/j.jcis.2009.05.054
[50] 孟涛. 浮选体系中颗粒对气泡运动及兼并行为的影响研究[D]. 中国矿业大学, 2019.
MENG T. Study on the influence of particles on bubble movement and coalescence behavior in flotation system [D]. China University of Mining and Technology, 2019.
[51] PAGUREVA N, TCHOLAKOVA S, RUSANOVA K, et al. Factors affecting the coalescence stability of microbubbles[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 508: 21−29.
[52] ZHANG Z, REN L, ZHANG Y. Role of nanobubbles in the flotation of fine rutile particles[J]. Minerals Engineering, 2021, 172: 107140. doi: 10.1016/j.mineng.2021.107140
[53] RAHMAN A, DARBAN A K, MAHMOUD A, et al. Nano−microbubble flotation of fine and ultrafine chalcopyrite particles[J]. International Journal of Mining Science and Technology, 2014, 24(4): 559−566. doi: 10.1016/j.ijmst.2014.05.021
[54] CAPPONI F, AZEVEDO A, OLIVEIRA H, et al. Column rougher flotation of fine niobium−bearing particles assisted with micro and nanobubbles[J]. Minerals Engineering, 2023, 199: 108119. doi: 10.1016/j.mineng.2023.108119
[55] TAO D, WU Z, AHMED S. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms[J]. Powder Technology, 2021, 379: 12−25. doi: 10.1016/j.powtec.2020.10.040
[56] CHEN G, REN L, ZHANG Y, et al. Improvement of fine muscovite flotation through nanobubble pretreatment and its mechanism[J]. Minerals Engineering, 2022, 189: 107868. doi: 10.1016/j.mineng.2022.107868
[57] TANG C, MA F, WU T, et al. Study on surface physical and chemical mechanism of nanobubble enhanced flotation of fine graphite[J]. Journal of Industrial and Engineering Chemistry, 2023, 122: 389−396. doi: 10.1016/j.jiec.2023.02.039
[58] LI C, ZHANG Y, ZHANG H. Study on removal of ultrafine graphite by nanobubbles−assisted flotation technique from graphite slime slurry[J]. Separation and Purification Technology, 2024, 328: 125079. doi: 10.1016/j.seppur.2023.125079
[59] ROSA A F, RUBIO J. On the role of nanobubbles in particle–bubble adhesion for the flotation of quartz and apatitic minerals[J]. Minerals Engineering, 2018, 127: 178−184. doi: 10.1016/j.mineng.2018.08.020
[60] CALGAROTO S, AZEVEDO A, RUBIO J. Flotation of quartz particles assisted by nanobubbles[J]. International Journal of Mineral Processing, 2015, 137: 64−70. doi: 10.1016/j.minpro.2015.02.010
[61] XIA Y, WANG L, ZHANG R, et al. Enhancement of flotation response of fine low−rank coal using positively charged microbubbles[J]. Fuel, 2019, 245: 505−513. doi: 10.1016/j.fuel.2019.02.092
[62] FAN M, TAO D, ZHAO Y, et al. Effect of nanobubbles on the flotation of different sizes of coal particle (Article)[J]. Minerals and Metallurgical Processing, 2013, 30(3): 157−161.
[63] SOBHY A, TAO D. Nanobubble column flotation of fine coal particles and associated fundamentals[J]. International Journal of Mineral Processing, 2013, 124: 109−116. doi: 10.1016/j.minpro.2013.04.016
[64] LI M, XU M, SUN L, et al. Effects of surface microbubbles on the adhesion between air bubble/oil droplet and graphite surfaces[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2023, 660: 130809. doi: 10.1016/j.colsurfa.2022.130809
[65] YOON R H, LUTTRELL G H. The effect of bubble size on fine particle flotation[J]. Mineral Processing and Extractive Metallurgy Review, 1989, 5: 101−122. doi: 10.1080/08827508908952646
[66] YOON R H, YORDAN J L. Induction time measurements for the quartz—amine flotation system[J]. Journal of Colloid and Interface Science, 1991, 141(2): 374−383. doi: 10.1016/0021-9797(91)90333-4
[67] YOON R H. The role of hydrodynamic and surface forces in bubble−particle interaction[J]. International Journal of Mineral Processing, 2000, 58(1/2/3/4): 129−143. doi: 10.1016/S0301-7516(99)00071-X
[68] 张凡凡. 浮选过程界面纳米气泡强化作用基础研究 [D]. 徐州: 中国矿业大学(徐州), 2023.
ZHANG F. ZHANG F F. Basic research on the strengthening effect of nanobubbles at the interface of flotation process [D]. Xuzhou: China University of Mining and Technology ( Xuzhou ), 2023.
[69] ANH VN, JAKUB N, JAN DM, et al. Attraction between hydrophobic surfaces studied by atomic force microscopy[J]. International Journal of Mineral Processing, 2003, 72(1/2/3/4): 215−225. doi: 10.1016/S0301-7516(03)00100-5
[70] DAI, FORNASIERO, RALSTON. Particle−bubble collision models − a review[J]. Advances in colloid and interface science, 2000, 85: 231−256. doi: 10.1016/S0001-8686(99)00030-5
[71] HAMPTON M A, NGUYEN A V. Nanobubbles and the nanobubble bridging capillary force[J]. Advances in Colloid and Interface Science, 2010, 154(1/2): 30−55. doi: 10.1016/j.cis.2010.01.006
[72] MEHDI A, ANH V N, GLEB E Y. Attractive forces between hydrophobic solid surfaces measured by AFM on the first approach in salt solutions and in the presence of dissolved gases (Article)[J]. Langmuir, 2015, 31(6): 1941−1949. doi: 10.1021/la504001z
[73] ATTARD P. Thermodynamic analysis of bridging bubbles and a quantitative comparison with the measured hydrophobic attraction[J]. Langmuir, 2000, 16(10): 4455−4466. doi: 10.1021/la991258+
[74] NAOYUKI I, YASUYUKI K, HIROBUMI U. Hydrophobic attraction between silanated silica surfacesin the absence of bridging bubbles[J]. Langmuir, 2012, 28(39): 13952−13959. doi: 10.1021/la303037d
[75] ESBEN T, ADAM C S, PER L H, et al. Force trace hysteresis and temperature dependence of bridging nanobubble induced forces between hydrophobic surfaces (Article)[J]. ACS Nano, 2008, 2(9): 1817−1824. doi: 10.1021/nn800218s
[76] PAUL K, LISA D, URS A P. Nanobubble enhanced agglomeration of hydrophobic powders[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2017, 530: 117−123.
[77] ZHOU W, NIU J, XIAO W, et al. Adsorption of bulk nanobubbles on the chemically surface−modified muscovite minerals[J]. Ultrasonics Sonochemistry, 2019, 51: 31−39. doi: 10.1016/j.ultsonch.2018.10.021
[78] WANG X, GAO P, LIU J, et al. Adsorption performance and mechanism of eco−friendly and efficient depressant galactomannan in flotation separation of chalcopyrite and molybdenite[J]. Journal of Molecular Liquids, 2021, 326: 115257. doi: 10.1016/j.molliq.2020.115257
[79] WANG X, YUAN S, LIU J, et al. Nanobubble−enhanced flotation of ultrafine molybdenite and the associated mechanism[J]. Journal of Molecular Liquids, 2022, 346: 118312. doi: 10.1016/j.molliq.2021.118312
[80] TAO T, LUTTRELL G H, YOON R H. A parametric study of froth stability and its effect on column flotation of fine particles[J]. International Journal of Mineral Processing, 2000, 59(1): 25−43.
[81] 韩子伟. 组合微泡发生器发泡性能研究 [D]. 昆明: 昆明理工大学, 2017.
HAN Z. Study on foaming performance of combined microbubble generator[D]. Kunming: Kunming University of Science and Technology, 2017.
[82] 王宾, 蒋昊. 浮选柱的研究与应用[J]. 中国有色金属学报, 2021, 31(4): 1027−1041. doi: 10.11817/j.ysxb.1004.0609.2021-36566
WANG B, JIANG H. Research and application of flotation column[J]. Chinese Journal of Nonferrous Metals, 2021, 31(4): 1027−1041. doi: 10.11817/j.ysxb.1004.0609.2021-36566
[83] 吴彬启, 李波, 薛立群. 旋流静态微泡浮选柱的应用[J]. 煤炭工程, 2011(S1): 84−85.
WU B Q, LI B, XUE L Q. Application of swirling static microbubble flotation column[J]. Coal Engineering, 2011(S1): 84−85.
[84] 王伟之, 李东林. 浮选柱技术的应用现状及发展趋势[J]. 有色金属(选矿部分), 2023(2): 19−29.
WANG W, LI D. Application status and development trend of flotation column technology[J]. Non−ferrous Metals (Beneficiation Part), 2023(2): 19−29.
[85] 陈新, 晁彦德, 黄业豪, 等. 旋流−静态微泡浮选柱在河南某微细粒钼矿的应用研究[J]. 矿产综合利用, 2023(5): 7−14.
CHEN X, CHAO Y, HUANG Y, et al. Application of cyclonic−static microbubble flotation column in a fine−grained molybdenum ore in Henan Province[J]. Multipurpose Utilization of Mineral Resources, 2023(5): 7−14.
[86] 吴荣, 石南南. 旋流−静态微泡浮选柱在某微细粒金矿浮选中的应用[J]. 现代矿业, 2017, 33(6): 124−127.
WU R, SHI N N. Application of cyclone−static microbubble flotation column in flotation of a micro−fine gold ore[J]. Modern Mining, 2017, 33(6): 124−127.
[87] 艾光华, 刘炯天, 曹亦俊, 等. 旋流−静态微泡浮选柱强化回收微细粒黑钨矿[J]. 中南大学学报(自然科学版), 2015, 46(11): 3983−3990.
AI G H, LIU J T, CAO Y J, et al. Strengthened recovery of fine wolframite by cyclonic−static microbubble flotation column[J]. Journal of Central South University (Science and Technology), 2015, 46(11): 3983−3990.
-



 下载:
下载: