Study on the Mechanism of Flotation Separation of Ilmenite and Olivine by Ternary Combination Collector based on Benzohydroxamic Acid
-
摘要:
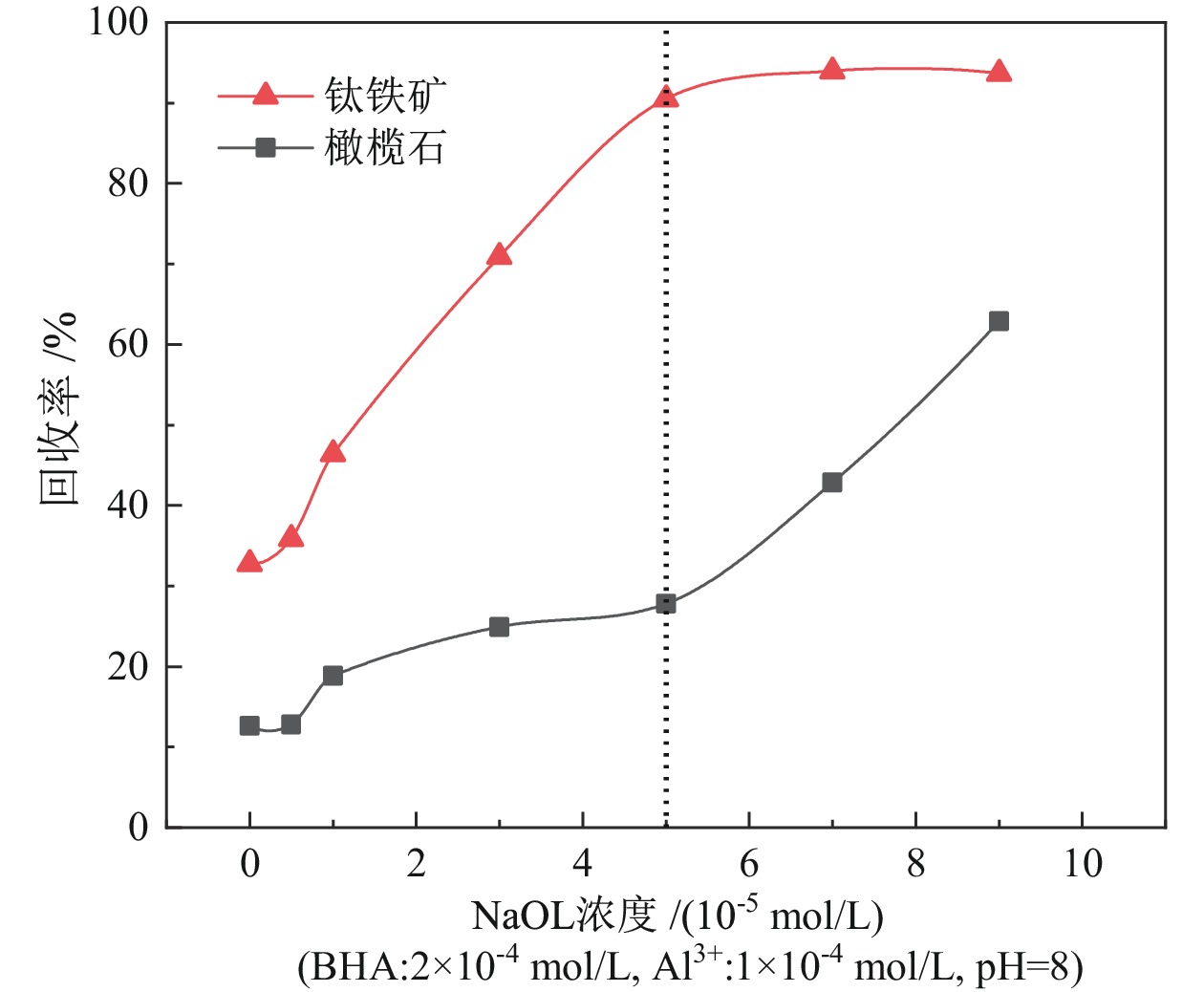
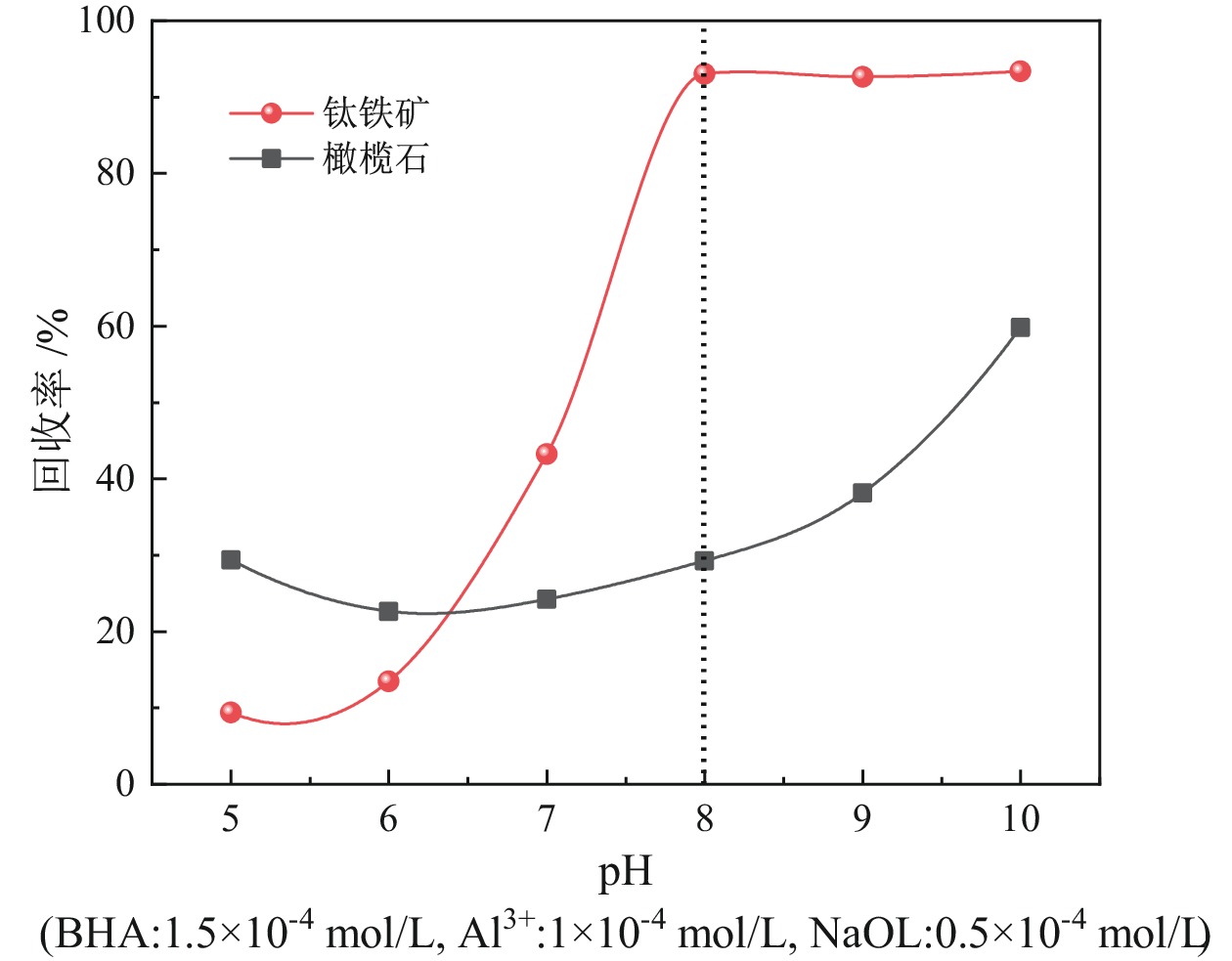
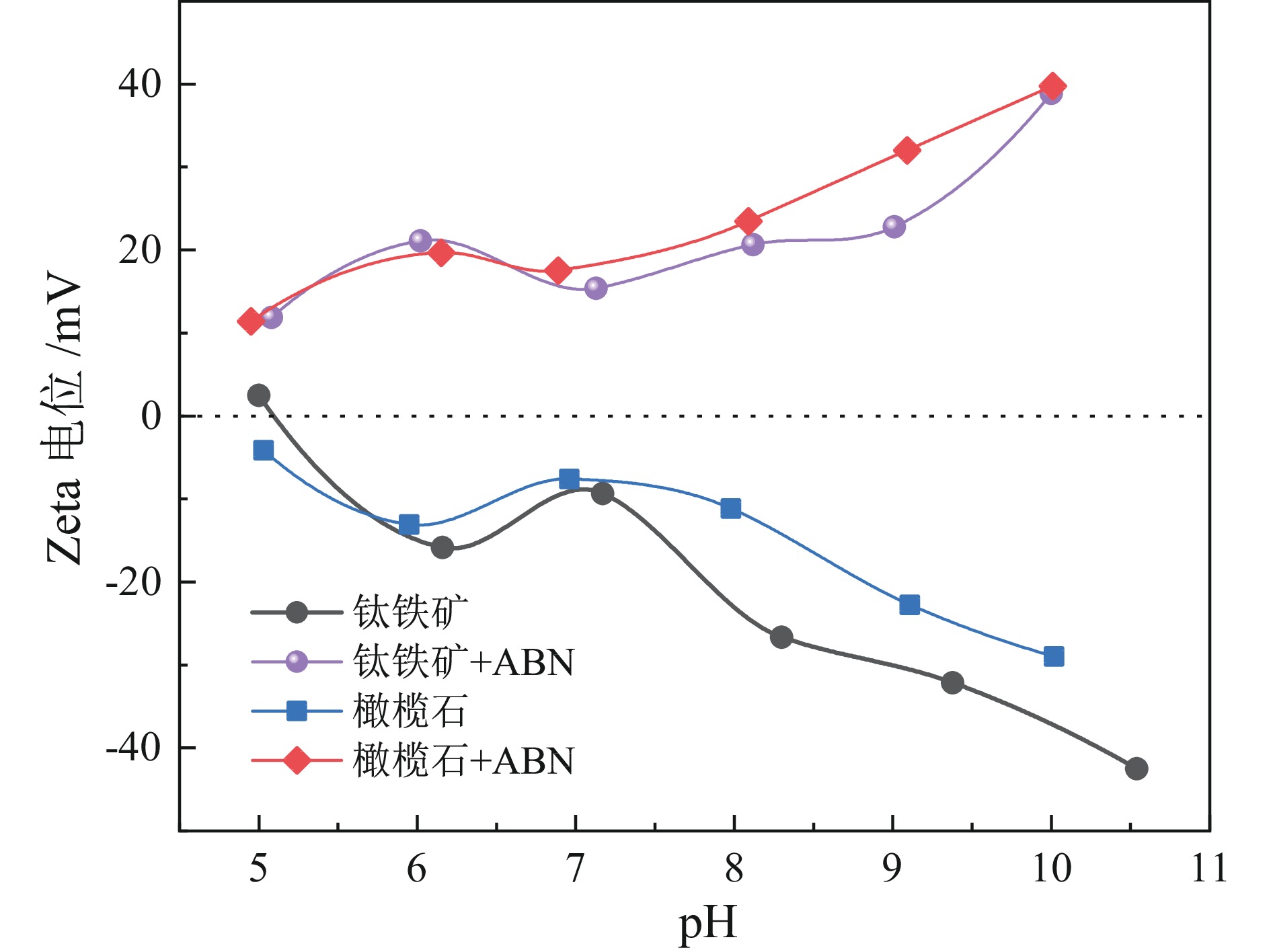
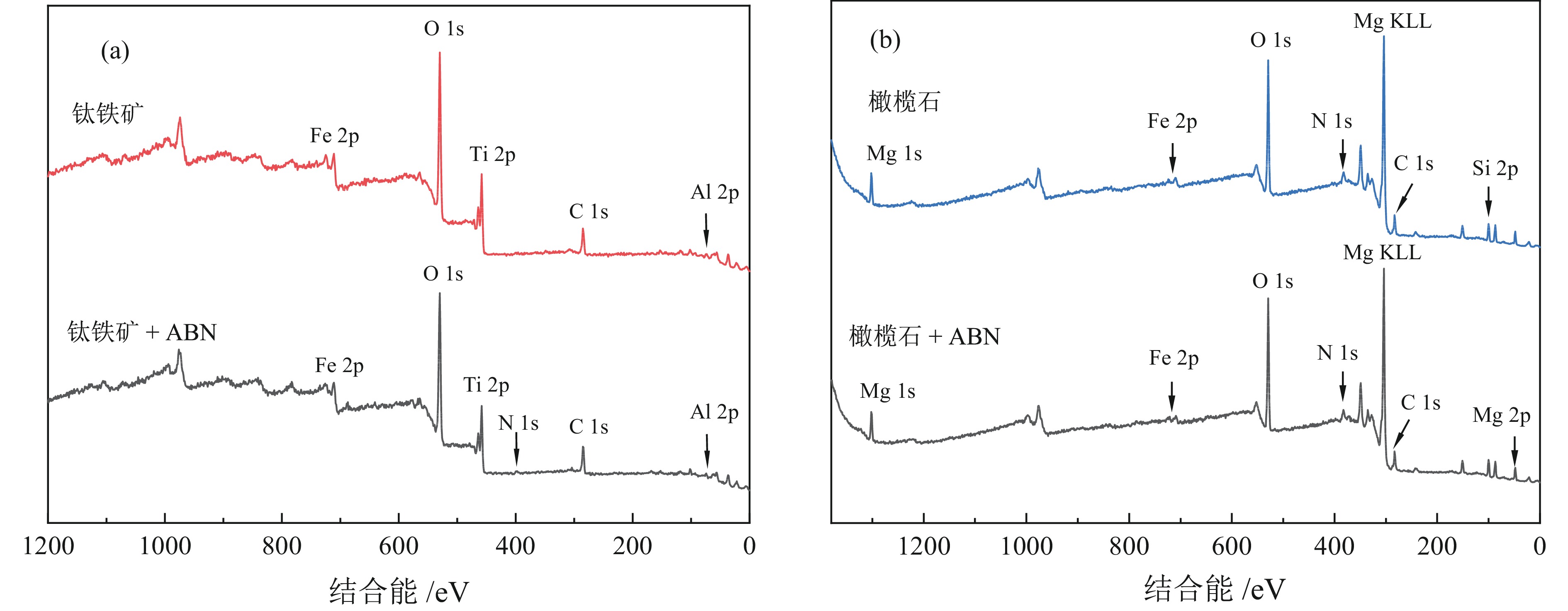
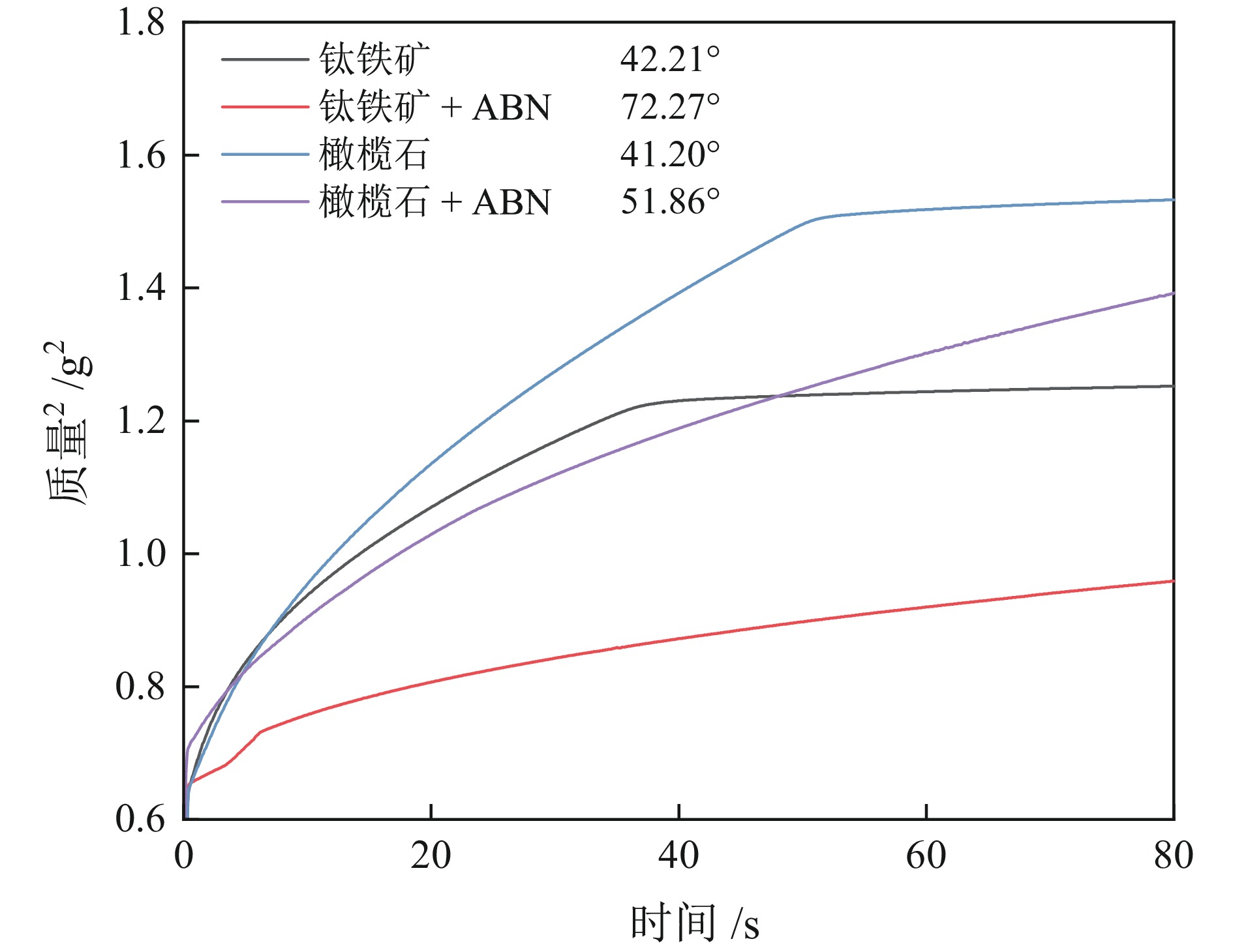
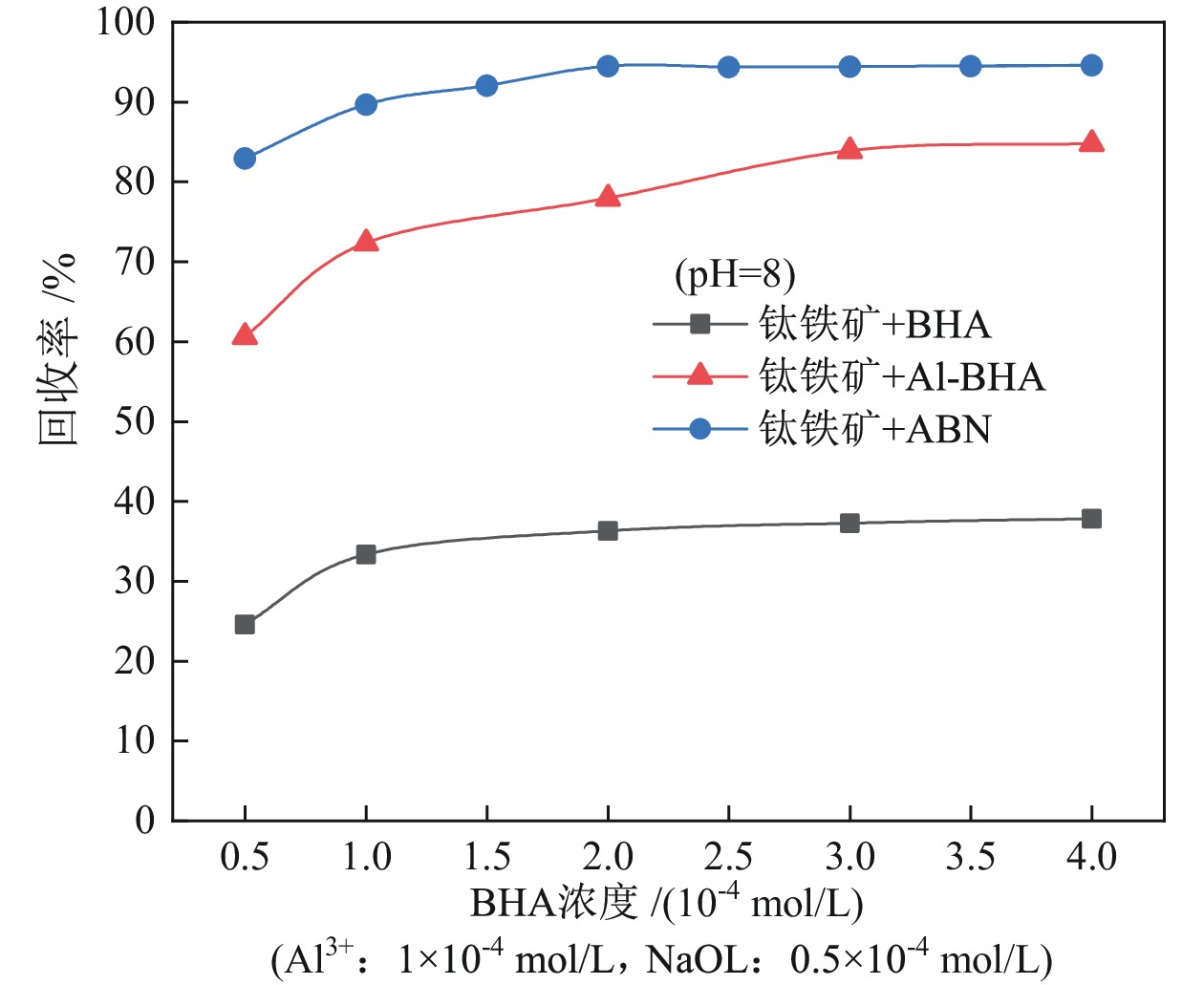
钛铁矿与橄榄石表面金属位点性质高度相似而难以分离。采用三氯化铝(Al)、苯甲羟肟酸(BHA)、油酸钠(NaOL)组装新型三元组合捕收剂(ABN),用于浮选分离钛铁矿和橄榄石。单矿物浮选实验表明,在Al、BHA和NaOL的摩尔比为2∶3∶1、pH值=8的条件下,钛铁矿回收率达90.4%,橄榄石回收率为25.9%。相比单一BHA,钛铁矿回收率提升了58.17百分点。表面Zeta电位测定和XPS分析表明, ABN在Ti(OH)、Fe(OH)位点发生化学吸附导致其表面电位正移了47.32 mV,在橄榄石表面只有少量电性吸附。粉末接触角测量结果显示,ABN使钛铁矿与橄榄石的表面接触角差异由1.01°提升至20.41°。ABN在钛铁矿表面的选择性吸附,增大了其与橄榄石的润湿性差异,利于实现两种矿物的浮选分离。
Abstract:The properties of metal sites on the surface of ilmenite and olivine are highly similar and are considered difficult to separate. A new ternary combined collector (ABN) was assembled using aluminum trichloride (Al), benzohydroxamic acid (BHA), and sodium oleate (NaOL) for the flotation separation of ilmenite and olivine. Single mineral flotation experiments showed that ilmenite was recovered at 90.4% and olivine at 25.9% under the condition of pH = 8 and a molar ratio of Al, BHA, and NaOL at 2∶3∶1. In comparison with the BHA system, the recovery rate of ilmenite was increased by 58.17%. Surface Zeta potential measurement and XPS analysis revealed that the chemical adsorption of ABN at Ti (OH) and Fe (OH) sites resulted in a positive shift of the surface potential by 47.32 mV, with only a small amount of electrical adsorption on the surface of olivine. The results of powder contact angle measurement indicated that ABN increased the surface contact angle difference between ilmenite and olivine from 1.01° to 20.41°. The selective adsorption of ABN on the surface of ilmenite led to an increased wettability difference between ilmenite and olivine, thereby facilitating the flotation separation of the two minerals.
-
Key words:
- ilmenite /
- olivine /
- flotation separation /
- combined collectors
-

-
表 1 钛铁矿和橄榄石单矿物的主要化学成分
Table 1. Chemical multielement analysis of ilmenite and olivine
/% 样品 TiO2 TFe SiO2 CaO MgO Al2O3 钛铁矿 50.12 25.36 1.86 0.21 0.09 0.56 橄榄石 0.02 8.47 43.52 0.04 47.23 0.12 表 2 钛铁矿和橄榄石表面元素浓度
Table 2. Relative concentration of elements on the ilmenite and olivine surface
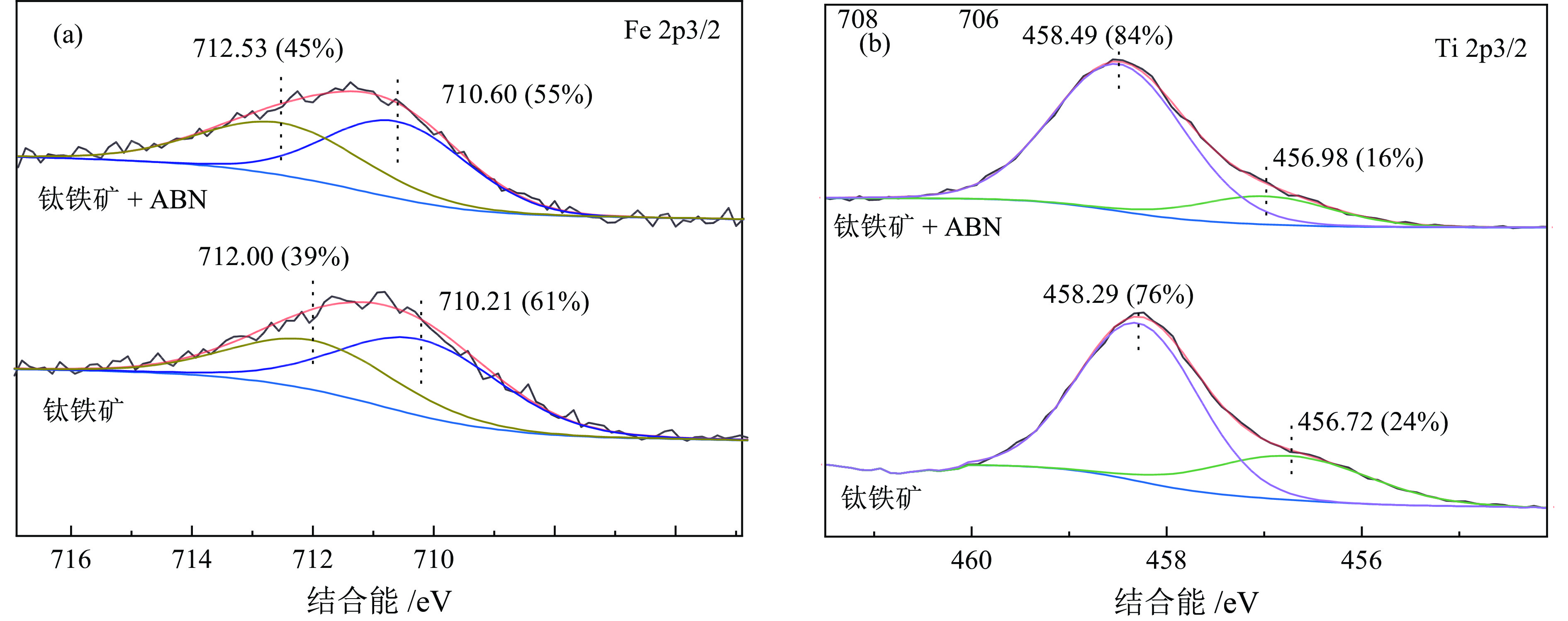
/% Samples C 1s O 1s Fe 2p Ti 2p Al 2p N 1s 钛铁矿 22.18 57.62 6.99 10.12 2.96 − 钛铁矿 + ABN 25.06 54.91 5.76 9.03 3.56 1.67 Samples C 1s O 1s Fe 2p Mg 2p Al 2p N 1s Si 2p 橄榄石 18.4 52.9 1.7 15.1 0.2 1.6 10.1 橄榄石 + ABN 18.5 53.3 1.1 15.3 0.6 1.4 9.6 -
[1] CHEN D S, SHENG ZHAO L S, QI T, et al. Desilication from titanium–vanadium slag by alkaline leaching[J]. Transactions of Nonferrous Metals Society of China, 2013, 23 (10): 3076−3082.
[2] AKBAR MEHDILO, MEHDI IRANNAJAD, BAHRAM REZAI. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 428: 111−119.
[3] RITESH PRAKASH, SUBRATA KUMAR MAJUMDER, ANUGRAH SINGH, et al. Flotation technique: Its mechanisms and design parameters[J]. Chemical Engineering and Processing−Process Intensification, 2018, 127: 249−270.
[4] 张松, 文书明, 刘建, 等. 微细粒钛铁矿选矿技术研究进展[J]. 矿产保护与利用, 2019, 39(1): 131−137.
ZHANG S, WEN S M, LIU J, et al. Research on mineral processing status of fine ilmenite[J]. Conservation and Utilization of Mineral Resources, 2019, 39(1): 131−137.
[5] 余攀, 丁湛, 李春龙, 等. 我国钛铁矿矿石浮选药剂研究进展[J]. 矿产保护与利用, 2020, 40(2): 82−87.
YU P, DING Z, LI C L, et al. Research progress on flotation agents of ilmenite in china[J]. Conservation and Utilization of Mineral Resources, 2020, 40(2): 82−87.
[6] ABHYARTHANA PATTANAIK, R. VENUGOPAL, Investigation of Adsorption mechanism of reagents (surfactants) system and its applicability in iron ore flotation – an overview[J]. Colloid and Interface Science Communications, 2018, 25: 41−65
[7] 高虎林, 刘建, 罗德强, 等. 钛铁矿和辉石浮选分离试验研究及抑制剂作用机理[J]. 矿产保护与利用, 2022, 42(1): 61−67+4.
GAO H L, LIU J, LUO D Q, et al. Experimental study on flotation separation of ilmenite and pyroxene and action mechanism of depressants[J]. Conservation and Utilization of Mineral Resources, 2022, 42(1): 61−67+4.
[8] ZHAI J H, CHEN P, SUN W, et al. A review of mineral processing of ilmenite by flotation[J]. Minerals Engineering, 2020, 157: 106558.
[9] LUO Y J, ZHANG G F, MAI Q Y, et al. Flotation separation of smithsonite from calcite using depressant sodium alginate and mixed cationic/anionic collectors[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 586: 124227.
[10] LI Y J, XIA W C, PENG Y L, et al. A novel coal tar−based collector for effective flotation cleaning of low rank coal[J]. Journal of Cleaner Production, 2020, 273: 123172.
[11] XU L, JIAO F, JIA W H, et al. Selective flotation separation of spodumene from feldspar using mixed anionic/nonionic collector[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 594: 124605.
[12] WEI Q, FENG L Q, DONG L Y, et al. Selective co−adsorption mechanism of a new mixed collector on the flotation separation of lepidolite from quartz[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 612: 125973.
[13] SHU K Q, XU L H, WU H Q, et al. In situ adsorption of mixed collectors BHA/DDA in spodumene−feldspar flotation system[J]. Separation and Purification Technology, 2020, 251: 117325.
[14] TIAN M J, ZHANG C Y, HAN H S, et al. Effects of the preassembly of benzohydroxamic acid with Fe (Ⅲ) ions on its adsorption on cassiterite surface[J]. Minerals Engineering, 2018, 127: 32−41.
[15] FANG S, XU L H, WU H Q, et al. Adsorption of Pb(Ⅱ)/benzohydroxamic acid collector complexes for ilmenite flotation[J]. Minerals Engineering, 2018, 126: 16−23.
[16] XIAO W, SHAO Y H, YU J Y, et al. Activation of ilmenite flotation by Al3+ in the benzohydroxamic acid (BHA) system[J]. Separation and Purification Technology, 2022, 299: 121770.
[17] YANG S Y, XU Y L, LIU C, et al. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 628: 127627.
[18] 肖玮, 邵延海, 尉佳怡, 等. 钛铁矿浮选药剂研究现状及展望[J]. 矿产保护与利用, 2021, 41(5): 160−167.
XIAO W, SHAO Y H, YU J Y, et al. Research status and prospect of ilmenite flotation reagents[J]. Conservation and Utilization of Mineral Resources, 2021, 41(5): 160−167.
[19] YANG Y H, XU L H, TIAN J, et al. Selective flotation of ilmenite from olivine using the acidified water glass as depressant[J]. International Journal of Mineral Processing, 2016, 156: 73−79.
[20] PARISA SEMSARI PARAPARI, MEHDI IRANNAJAD, AKBAR MEHDILO. Effect of acid surface dissolution pretreatment on the selective flotation of ilmenite from olivine and pyroxene[J]. International Journal of Mineral Processing, 2017, 167: 49−60.
[21] XIAO W, SHAO Y H, YU J Y, et al. Adsorption differences and mechanism of Pb−BHA and Al−BHA in the flotation separation of ilmenite and titanaugite[J]. Minerals Engineering, 2023, 197: 108072.
[22] LI J H, SHAO Y H, XIAO W, et al. Novel insights into the microstructure of Al−BHA on the surface of ilmenite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 677: 132341.
[23] WANG J J, GAO Z Y, HAN H S, et al. Impact of NaOL as an accelerator on the selective separation of scheelite from fluorite using a novel self−assembled Pb−BHA−NaOL collector system[J]. Applied Surface Science, 2021, 537: 147778.
[24] S. P. MAGALHÃES DA SILVA, J. M. OLIVEIRA, Cork powders wettability by the Washburn capillary rise method[J]. Powder Technology, 2021, 387: 16−21.
[25] YUAN Z T, ZHAO X, MENG Q Y, et al. Adsorption mode of sodium citrate for achieving effective flotation separation of ilmenite from titanaugite[J]. Minerals Engineering, 2021, 171: 107086.
[26] FANG S, XU L H, WU H Q, et al. Influence of aluminum–sodium silicate on olivine flotation with sodium oleate[J]. Minerals Engineering, 2019, 143: 106008.
-



 下载:
下载: