An Improved Urea Adduction Method for Analyzing Carbon Isotope of ppm-level n-alkanes in Soil and Plant Samples
-
摘要:
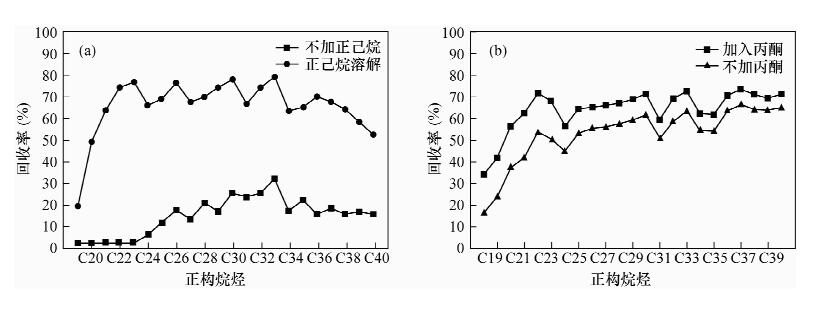
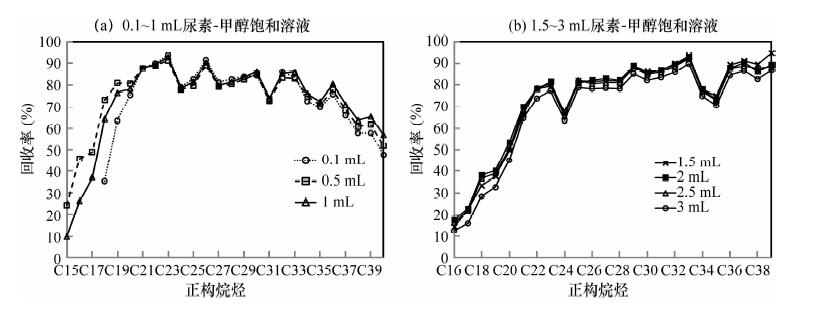
尿素络合法和5Å分子筛法是常用的分离富集环境样品中正构烷烃的方法, 但由于复杂的处理流程对于低含量正构烷烃的回收率普遍较低。本文通过优化尿素络合法分离富集正构烷烃的实验条件, 建立了尿素络合法分离-气相色谱/同位素质谱分析土壤和植物中低含量正构烷烃单体碳同位素的方法。即采用正己烷-丙酮溶解样品, 在4℃冰箱中与2 mL尿素-甲醇饱和溶液反应48 h; 用去离子水溶解尿素络合物, 加入正己烷后分离出有机相和水相, 分别用正己烷、丙酮-正己烷萃取有机相和水相中的正构烷烃。中长链正构烷烃的回收率达79%~104%, 高于5Å分子筛法和已有尿素络合法的富集效果; 单体碳同位素的分析精度为0.09‰~0.63‰(1σ)。利用改进的方法分析实际样品, 大幅降低了未分峰和共流出物的干扰, 提高了实际样品中ppm级中长链正构烷烃的回收率。
Abstract:Both the urea adduction method and 5Å molecular sieves are used to separate straight chains from branched/cyclic hydrocarbons, however, the recovery of the low concentration of n-alkanes are generally low due to the complex process. Through optimizing experimental conditions of urea adduction method to separate n-alkanes, a method of Gas Chromatography/Isotope Mass Spectrometry to the determination of low concentration of n-alkanes in soil and plants was developed. Hexane and acetone are used to dissolve a saturated fraction. The mixture was added to a 2 mL urea-saturated methanol solution and allowed to stand for 48 h at 4℃. After the urea crystals were dissolved in distilled water, hexane was added into the mixture to form aqueous and organic phases. The n-alkanes were recovered by hexane in aqueous phase and by a mixture of hexane and acetone in organic phases. The recoveries of middle-and long-chain n-alkanes ranged from 79% to 104%, higer than the recoveries by the urea adduction method and 5Å molecular sieves. The precision of single carbon isotope ranged from 0.09‰ to 0.63‰ (1σ). Using the proposed method, the interferences of undivided peak and total outflow were significantly reduced, the recoveries of middle-and long-chain n-alkanes with ppm level in actual samples.
-

-
表 1 尿素络合法分离正构烷烃的回收率、精密度;尿素络合法与5Å分子筛法分析正构烷烃单体碳同位素值对比
Table 1. An Improved Urea Adduction Method for Analyzing Carbon Isotope of ppm-level n-alkanes in Soil and Plant Samples by Gas Chromatography-Isotope Mass Spectrometry
组分名 尿素络合法 5Å分子筛法 正构烷烃的络合分离回收率(%) 正构烷烃的δ13C(%) 正构烷烃的δ13C(%) 1 2 3 4 5 6 平均值 RSD
(%)1 2 3 4 5 6 平均值 1σ 平均值 C19 86 90 90 92 87 89 89 2 -30.12 -30.55 -30.36 -30.12 -30.77 -30.33 -30.37 0.25 -30.61 C20 84 87 85 89 82 84 85 3 -32.44 -32.06 -31.79 -31.72 -31.63 -31.11 -31.79 0.45 -31.84 C21 92 93 95 96 89 93 93 3 -28.93 -28.93 -28.55 -28.84 -28.66 -28.63 -28.76 0.17 -29.17 C22 94 93 95 96 91 94 94 2 -33.94 -33.98 -34.29 -34.26 -34.10 -33.46 -34.01 0.30 -34.09 C23 90 97 98 100 93 97 96 4 -32.59 -32.64 -32.2 -32.49 -32.70 -33.11 -32.62 0.30 -32.9 C24 79 80 81 82 77 80 80 2 -31.77 -32.26 -32.76 -31.94 -31.98 -32.12 -32.14 0.34 -32.4 C25 84 84 85 86 81 85 84 2 -30.36 -30.77 -30.52 -30.55 -30.27 -30.56 -30.50 0.17 -30.35 C26 92 94 94 96 89 94 93 2 -32.78 -32.24 -33.03 -32.06 -32.29 -31.74 -32.36 0.47 -32.48 C27 84 85 85 85 80 84 84 3 -30.85 -31.00 -31.27 -30.82 -31.07 -31.52 -31.09 0.27 -30.94 C28 83 86 85 86 80 85 84 3 -30.27 -30.34 -30.01 -30.09 -29.96 -30.00 -30.11 0.16 -30.76 C29 87 89 88 90 83 88 87 3 -30.71 -30.55 -30.54 -30.73 -30.58 -30.72 -30.64 0.09 -30.83 C30 89 90 91 93 84 91 90 3 -32.72 -32.58 -32.06 -32.34 -32.43 -32.09 -32.37 0.26 -32.1 C31 79 80 80 81 75 79 79 3 -31.53 -31.90 -31.99 -31.84 -31.72 -31.71 -31.78 0.16 -31.88 C32 92 94 93 94 87 94 92 3 -30.43 -30.63 -30.19 -30.80 -30.56 -30.21 -30.47 0.24 -30.91 C33 95 97 96 97 90 96 95 3 -34.01 -33.81 -34.67 -34.10 -33.54 -33.46 -33.93 0.44 -33.7 C34 88 90 89 91 84 89 89 3 -30.6 -30.23 -31.74 -30.89 -31.05 -30.94 -30.91 0.50 -31.18 C35 88 90 90 91 84 90 89 3 -30.83 -30.84 -30.37 -30.85 -30.89 -29.72 -30.58 0.47 -30.66 C36 103 106 104 106 99 104 104 3 -30.19 -29.20 -30.16 -30.11 -29.13 -29.93 -29.79 0.49 -29.09 C37 101 104 103 106 98 103 102 3 -30.17 -29.99 -29.24 -29.42 -30.90 -30.49 -30.04 0.63 -29.82 C38 88 92 90 93 87 90 90 3 -29.21 -29.49 -29.50 -29.41 -29.71 -29.46 -29.47 0.16 -29.91 C39 88 97 94 98 91 95 94 4 -29.35 -29.20 -28.57 -28.68 -28.96 -28.83 -28.93 0.30 -29.14 C40 73 85 84 87 83 85 83 6 -35.10 -35.12 -35.49 -34.54 -35.29 -34.76 -35.05 0.35 -34.77 表 2 实际样品中正构烷烃的络合分离回收率及正构烷烃单体碳同位素分析结果
Table 2. The recoveries and accuracies of compound specific carbon isotopes of n-alkanes in practical sample
组分名 正构烷烃的回收率(%) 正构烷烃的δ13C(‰) 抚仙湖
土壤上海
土壤松树 银杏 小叶
冬青龙爪槐 抚仙湖
土壤上海
土壤松树 银杏 小叶
冬青龙爪槐 C16 60.4 56.9 - - - - -28.74 -27.44 - - - - C17 65.9 70.2 - 77.7 39.6 - -29.33 -30.03 - -27.83 - - C20 95.3 99.2 - - 99.4 - -27.62 -27.84 - - - - C21 81.4 76.2 - 86.1 105.2 - -31.39 -32.96 - - - - C22 80.4 70.7 83.9 94.9 71.9 88.1 -33.19 -34.59 - -29.17 - -35.58 C23 53.8 81.0 80.7 89.2 94.7 84.6 -29.67 -31.33 -28.67 -29.11 -28.73 -34.06 C24 - 73.4 82.8 92.7 97.7 88.7 - -31.25 -28.26 -29.82 -28.74 -35.67 C25 75.6 84.6 82.5 89.6 88.1 86.4 -29.69 -31.45 -29.42 -30.05 -28.35 -36.46 C26 85.6 76.8 96.1 92.0 104.5 91.9 -30.68 -31.04 -27.65 -30.11 -28.17 -38.33 C27 79.5 95.8 82.5 88.5 89.1 86.1 -28.48 -32.30 -30.14 -30.76 -28.54 -37.53 C28 33.4 89.5 81.2 85.1 87.0 83.6 -31.51 -32.15 -29.63 -30.08 -29.43 -37.61 C29 80.3 106.6 79.4 85.4 87.4 85.3 -30.66 -33.29 -30.62 -30.11 -28.60 -35.49 C30 75.2 79.1 75.1 83.8 84.4 82.5 -32.27 -33.52 -33.58 -31.95 -31.35 -39.77 C31 78.6 103.0 73.7 89.6 84.3 85.5 -31.00 -32.60 -29.74 -29.42 -29.45 -34.60 C32 74.3 71.8 61.6 - 79.7 80.2 -32.50 -34.33 - - - -38.70 C33 68.0 72.3 61.0 - 72.6 77.8 -27.60 -30.47 -27.86 - - -34.40 C34 96.6 - - - - 103.2 -26.89 - - - - -33.67 C35 61.8 42.0 41.4 - - - -21.50 -26.17 - - - - -
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] O'Leary M H. Biochemical Basis of Carbon Isotope Fractionation[M]// Ehleringer J R, Hall A E, Farquhar G D. Stable Isotopes and Plant Carbon-water Relations. San Diego: Academic Press, 1993: 19-28.
[12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] 王素萍. 青藏高原东北部湖泊沉积物末次冰消期以来正构烷烃分子分布特征及其碳、氢同位素的古环境意义[D]. 兰州: 兰州大学, 2011.
Wang S P. Molecular Distribution and C-H Isotopes of n-alkanes of Lacustrine Sediments since the Last Deglaciation in the Northeast Tibet Plateau[D]. Lanzhou: Lanzhou University, 2011.
[29] 王彦美. 松辽盆地南部上白垩统烃源岩和原油中正构烷烃的碳氢同位素组成研究[D]. 广州: 中国科学院广州地球化学研究所, 2006.
Wang Y M. C and H Isotopic Compositions of n-alkanes in Crude Oils and Extracts of the Upper Cretaceous from the Southern Songliao Basin[D]. Guangzhou: Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 2006.
[30] [31] [32] -



 下载:
下载: