The Preparation and Performance of SPE Packing of Bonded Ligand on the Surface of Nanometer Titanium Dioxide
-
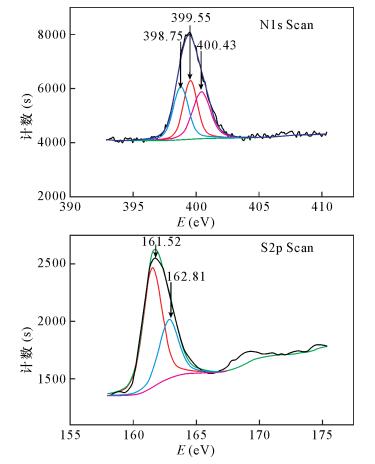
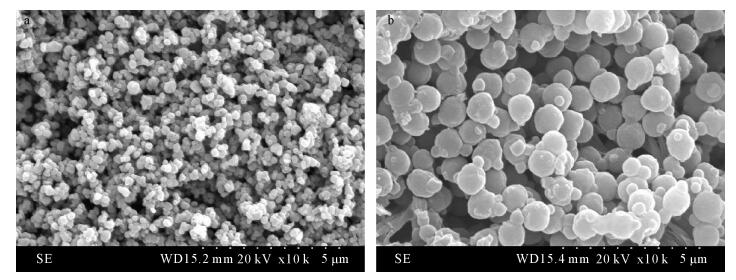
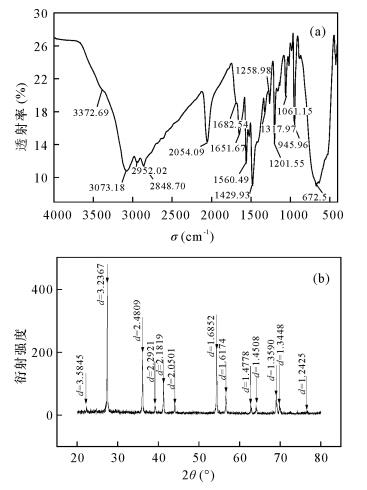
摘要: 有机配位体/无机纳米复合材料作为固相萃取填料用于重金属离子分离富集是当前分析化学研究的热点课题。本文将含有N、S配位原子的氨基硫脲通过缩合反应接枝于纳米二氧化钛表面,制备了一种新型纳米TiO2/TSC复合固相萃取填料。通过红外光谱、X射线衍射、X射线光电子能谱和扫描电镜表征,此填料与共混法制备的聚合物包覆纳米二氧化钛复合填料相比,二氧化钛粒子(尺寸200~300 nm)分布更均匀,结构更稳定。用该填料制备的固相萃取小柱静态吸附Sb3+、Cd2+和Ba2+在30℃时饱和吸附量分别为13.9 mg/g、12.9 mg/g和11.2 mg/g,在优化的实验条件下三种金属离子的吸附回收率分别达到97.94%、95.65%和94.04%,实验数据重现性高(RSD < 5.5%),吸附性能优于聚苯乙烯-甲基丙烯醛-氨基硫脲包覆纳米二氧化钛和纳米二氧化钛两种填料。本填料结合ICP-MS测定水样中以上三种离子的检出限分别为0.061 μg/L、0.013 μg/L和0.075 μg/L。Abstract: At present, the solid phase extraction packing of organic ligand/nano inorganic composite material is used to separate and enrich metal ions, which has become a hot research topic in analytical chemistry. A novel nano-TiO2/TSC solid-phase extraction packing was prepared by grafting thiosemicarbazone containing N, S coordination atoms onto the surface of nano-TiO2 by condensation reaction and is described in this paper. The structures and the property of synthesized composite were characterized and analyzed by Infrared Spectroscopy, X-ray Diffraction Scanning, X-ray Photoelectron Spectroscopy, and Electron Microscopy. The composition and size distribution of the packing particle were more uniform (the particle size is 200-300 nm) and more stable than the solid phase extraction packing made by physical blending technology. The saturation adsorption capacities of Sb3+, Cd2+ and Ba2+ on the solid phase extraction column prepared by this method at 30℃ were 13.9 mg/g, 12.9 mg/g, and 11.2 mg/g, respectively. The recoveries of three metal ions were 97.94%, 95.65% and 94.04%, respectively under the optimal experimental conditions. The data has good reproducibility with RSD < 5.5%. Thus, the adsorption property of the new nanometer TiO2/TSC composite solid phase extraction packing is an improvement on the styrene-methyl acrolein-thiosemicarbazide coated nano-titanium dioxide packing and nano-titanium dioxide packing. The contents of Sb3+, Cd2+ and Ba2+ in water samples were determined by Inductively Coupled Plasma-Mass Spectrometry coupled with the TiO2/TSC composite solid phase extraction packing, which yields detection limits of 0.061 μg/L, 0.013 μg/L, and 0.075 μg/L for Sb3+, Cd2+ and Ba2+, respectively.
-
Key words:
- nano-titanium dioxide /
- bond ligand /
- Solid Phase Extraction /
- adsorption property /
- heavy metals
-

-
表 1 流速对金属离子回收率的影响
Table 1. The influence of velocity on recovery rate of metal ions
金属离子 不同流速下金属离子的回收率(%) 0.5 mL/min 1.0 mL/min 1.5 mL/min 2.0 mL/min Sb3+ 97.94 96.03 92.18 85.43 Cd2+ 95.65 94.44 90.57 86.74 Ba2+ 94.04 93.41 90.56 85.05 表 2 洗脱剂对金属离子洗脱率的影响
Table 2. The influence of the elution liquid on recovery rate of metal ions
洗脱剂 金属离子回收率(%) Sb3+ Cd2+ Ba2+ 10 mL 1 mol/L硝酸 88.45 89.92 90.94 10 mL 3 mol/L硝酸 92.46 91.54 93.26 10 mL 5 mol/L硝酸 95.03 96.05 96.72 10 mL 1 mol/L硝酸+0.25 mL三乙醇胺 91.78 92.65 92.82 10 mL 3 mol/L硝酸+0.25 mL三乙醇胺 94.43 96.91 96.09 10 mL 5 mol/L硝酸+0.25 mL三乙醇胺 98.43 98.28 99.07 表 3 固相萃取填料的吸附性能对比
Table 3. A comparison of adsorption performance of the SPE packings
填料 回收率(%) RSD(%) Sb3+ Cd2+ Ba2+ Sb3+ Cd2+ Ba2+ 表面键合配位体二氧化钛 97.94 95.65 94.04 5.4 4.7 5.1 聚合物包覆纳米二氧化钛 96.87 94.23 93.67 10.2 11.6 9.9 纳米二氧化钛 88.33 85.26 86.84 11.5 9.3 8.8 表 4 样品测定结果(n=6)及检出限(n=20)
Table 4. Analytical results (n=6) and detection limits (n=20) of the sample
样品 Sb3+测定值(μg/L) Cd2+测定值(μg/L) Ba2+测定值(μg/L) 江水 0.42 0.23 2.56 湖水 1.53 3.01 10.6 地下水 0.11 0.14 0.87 加标回收率(%) 97.6~106.0 98.8~103.0 99.2~101.0 检出限(μg/L) 0.061 0.013 0.075 -
[1] 曹斌, 何松洁, 夏建新.重金属污染现状分析及其对策研究[J].中央民族大学学报(自然科学版), 2009, 18(1):29-33. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=zymz200901008&dbname=CJFD&dbcode=CJFQ
Cao B, He S J, Xia J X.Heavy metal pollution present situation analysis and countermeasure research[J].Journal of Central National University (Natural Science), 2009, 18(1):29-33. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=zymz200901008&dbname=CJFD&dbcode=CJFQ
[2] 付海曦, 刘威, 张春辉, 等.水体中重金属离子的检测方法研究进展[J].理化检验(化学分册), 2012, 48(4):496-499. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=lhjh201204045&dbname=CJFD&dbcode=CJFQ
Fu H X, Liu W, Zhang C H, et al.Advances in detection of heavy metal ions in water bodies[J].Physical Testing and Chemical Analysis (Part B:Chemical Analysis), 2012, 48(4):496-499. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=lhjh201204045&dbname=CJFD&dbcode=CJFQ
[3] 袁敏, 武建超, 于劲松, 等.水中重金属检测方法的研究进展[J].应用化工, 2015, 44(4):724-728. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=sxhg201504037&dbname=CJFD&dbcode=CJFQ
Yuan M, Wu J C, Yu J S, et al.Research progress of heavy metal detection in water[J].Applied Chemical Industry, 2015, 44(4):724-728. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=sxhg201504037&dbname=CJFD&dbcode=CJFQ
[4] 石小飞, 让蔚清.固相萃取技术分离富集食品和环境水中铅的应用进展[J].理化检验(化学分册), 2013, 49(5):622-627. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=lhjh201305038&dbname=CJFD&dbcode=CJFQ
Shi X F, Rang W Q.The application of solid phase extraction technology to separate enriched food and lead in environmental water[J].Physical Testing and Chemical Analysis (Part B:Chemical Analysis), 2013, 49(5):622-627. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=lhjh201305038&dbname=CJFD&dbcode=CJFQ
[5] 郭明, 武晓鹏, 孙东海, 等.新型基质固相萃取重金属离子分析及残留关联性[J].浙江农林大学学报, 2012, 29(4):551-557. http://d.wanfangdata.com.cn/Periodical_zjlxyxb201204011.aspx
Guo M, Wu X P, Sun D H, et al.New matrix solid phase extraction of heavy metal ion analysis and residual correlation[J].Journal of Zhejiang Agricultural and Forestry University, 2012, 29(4):551-557. http://d.wanfangdata.com.cn/Periodical_zjlxyxb201204011.aspx
[6] Kiptoo J K, Ngila J C, Silavwe N D.Solid-phase extraction of Zn(Ⅱ), Cu(Ⅱ), Ni(Ⅱ) and Pb(Ⅱ) on poly(vinyl chloride) modified with 3-ferrocenyl-3-hydroxydithioacrylic acid, and their subsequent determination by ectrothermal atomic absorption spectrometry[J].Microchimica Acta, 2008, 160(1):211-218. http://link.springer.com/article/10.1007/s00604-007-0831-y
[7] Hu B, He M, Chen B B.Nanometer-sized materials for solid phase extraction of trace elements[J].Analytical and Bioanalytical Chemistry, 2015, 407:2685-2710. doi: 10.1007/s00216-014-8429-9
[8] 董惟昕, 张光华, 朱军峰.螯合树脂对金属离子吸附性能及应用的研究进展[J].陕西科技大学学报, 2010, 28(2):96-99. http://www.doc88.com/p-490333098704.html
Dong W X, Zhang G H, Zhu J F.Research progress of chelating resin on the properties and applications of metal ion adsorption[J].Journal of Shaanxi University of Science and Technology, 2010, 28(2):96-99. http://www.doc88.com/p-490333098704.html
[9] Yavuz E, Tokalıoǧlu Ş, Erkılıç H, et al.Novel chelating resin for solid-phase extraction of metals in certified reference materials and waters[J].Analytical Letters, 2017, 50(2):364-378. doi: 10.1080/00032719.2016.1181643
[10] Pei L, Yong C Q, Bin H, et al.Study of the adsorption behavior of heavy metal ions on nanometer-size titanium dioxide with ICP-AES[J].Fresenius Journal of Analytical Chemistry, 2000, 368(6):638-640. doi: 10.1007/s002160000546
[11] Demina P A, Kuzmichev A N, Tsybinsky A M.Synthe-sis, characterization and adsorption behavior of Mo(Ⅵ) and W(Ⅵ) ions on titanium dioxide nanoparticles containing anatase modification[J].Applied Nanoscience, 2014, 4(8):979-987. doi: 10.1007/s13204-013-0279-9
[12] 刘正华, 周方钦, 黄荣辉, 等.纳米二氧化钛对痕量铅的吸附性能研究[J].分析试验室, 2006, 25(11):63-65. doi: 10.3969/j.issn.1000-0720.2006.11.015
Liu Z H, Zhou F Q, Huang R H, et al.Nanoscale titanium dioxide adsorption performance of trace lead[J].Analytical Laboratory, 2006, 25(11):63-65. doi: 10.3969/j.issn.1000-0720.2006.11.015
[13] 刘艳, 梁沛, 郭丽, 等.负载型纳米二氧化钛对重金属离子吸附性能的研究[J].化学学报, 2005, 63(4):312-316. http://www.cqvip.com/QK/91047X/2005004/11917801.html
Liu Y, Liang P, Guo L, et al.Study on the adsorption properties of heavy metal ions by load-type nano-TiO2[J].Journal of Chemistry, 2005, 63(4):312-316. http://www.cqvip.com/QK/91047X/2005004/11917801.html
[14] 丁延伟, 范崇政.纳米二氧化钛表面包覆的研究[J].现代化工, 2001, 21(7):18-22. https://www.wenkuxiazai.com/doc/268d3526af45b307e87197e9-3.html
Ding Y W, Fan C Z.Study on surface coating of nano titanium dioxide[J].The Modern Chemical Industry, 2001, 21(7):18-22. https://www.wenkuxiazai.com/doc/268d3526af45b307e87197e9-3.html
[15] 侯冬枝, 谢长生.纳米二氧化钛的表面修饰与应用的研究进展[J].材料导报, 2003, 17(9):89-91. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cldb2003z1029
Hou D Z, Xie Z S.Research progress in surface modification and application of nano titanium dioxide[J].Material Review, 2003, 17(9):89-91. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cldb2003z1029
[16] Wu W, Lu S C, Chen J F.Grafting modification on the surface of titanium dioxide by polystyrene[J].Journal of University of Science and Technology Beijing, 2003, 10(6):52-56. http://en.cnki.com.cn/Article_en/CJFDTOTAL-BJKY200306011.htm
[17] 申书昌, 刘静禹, 李晶.聚苯乙烯-丙烯醛-氨基硫脲/纳米二氧化钛复合固相萃取填料的制备及其性能[J].分析试验室, 2017, 36(4):471-476. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=fxsy201704022&dbname=CJFD&dbcode=CJFQ
Shen S C, Liu J Y, Li J.Preparation and properties of polystyrene-propylene aldehyde-amino thiourea/nano-TiO2 composite solid phase extraction[J].Analytical Laboratory, 2017, 36(4):471-476. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=fxsy201704022&dbname=CJFD&dbcode=CJFQ
[18] 许芳, 张利平, 程先忠, 等.改性桑树叶吸附材料对废水中Cd(Ⅱ)的吸附性能研究[J].岩矿测试, 2016, 35(1):62-68. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.01.011
Xu F, Zhang L P, Cheng X Z, et al.Study on biosorption performance of Cd(Ⅱ) in waste water by modified mulberry leaves[J].Rock and Mineral Analysis, 2016, 35(1):62-68. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.01.011
[19] Jazi M B, Arshadi M, Amiri M J, et al.Kinetic and thermodynamic investigations of Pb(Ⅱ) and Cd(Ⅱ) adsorption on nanoscale organo-functionalized SiO2-Al2O3[J].Journal of Colloid and Interface Science, 2014, 422(1):16-24. http://www.whxb.pku.edu.cn/EN/abstract/abstract29100.shtml
[20] 赵秀琴.软硬酸碱理论的一些应用[J].安庆师范学院学报(自然科学版), 1998, 4(4):86-87. http://www.cnki.com.cn/Article/CJFDTotal-AQSX804.028.htm
Zhao X Q.Some applications of the theory of soft and hard acids[J].Journal of Anqing Normal University (Natural Science), 1998, 4(4):86-87. http://www.cnki.com.cn/Article/CJFDTotal-AQSX804.028.htm
-



 下载:
下载: