Rapid Determination of Inorganic and Organic Iodine in Edible Salt and Kelp Samples by a Z-Ⅱ Iodine Probe
-
摘要: 目前食盐和海带产品中碘含量分析主要是总碘含量测定,而无机碘与有机碘形态分析主要依靠大型精密仪器联用技术。本文研制了一种基于硫化碘化银法研制的全固态、无内参液的晶体膜电极(Z-Ⅱ碘探针),采用聚四氟乙烯消化罐微波消解技术,建立了食用盐及海带中的无机碘及有机碘含量测定方法。实验结果表明:食盐中无机碘含量为20mg/kg,有机碘含量为2mg/kg,海带中有机碘含量为2.9×103mg/kg。无机碘及有机碘含量分别在10~100mg/kg范围内线性关系良好,加标回收率为97.1%~101.0%,检出限均为1mg/kg。本方法应用于食盐和海带实际样品中的碘含量测定,无机碘和有机碘测定结果的相对标准偏差(RSD)分别小于0.92%和2.49%,且测定结果与国家标准方法和紫外分光光度法的测定结果基本一致。该方法具有试剂耗量少、成本低的特点,操作方便,可应用于有机碘和无机碘含量的测定。Abstract:
BACKGROUNDAt present, iodine content in edible salt and kelp products is mainly determined as the form of total iodine content, but the speciation analysis of inorganic iodine and organic iodine mainly relies on the hyphenated techniques of large-scale instruments. OBJECTIVESTo establish the determination method of iodine content in edible salt and kelp as well as the digestion method for the organic iodine in the closed PTFE bomb. METHODSRapid determination of inorganic and organic iodine content in edible salt and kelp products by using crystal membrane electrode technique with a solid-state and no internal reference liquid crystal film electrode (Z-Ⅱ iodine probe) developed by silver sulfide iodide method, combined with PTFE bomb microwave digestion technique. RESULTSTable salt contained 20mg/kg of inorganic iodine, and 2mg/kg of organic iodine. The concentration of organic iodine was 2.9×103mg/kg in kelp. The linear correlation of inorganic and organic iodine contents in the range of 10-100mg/kg was good. The standard-addition recoveries were 97.1%-101.0%, and the detection limit was up to 1mg/kg. This method was applied to the determination of iodine content in actual edible salt and kelp on the market, the relative standard deviation of inorganic and organic iodine in the samples was less than 0.92% and 4.20%, respectively. The analytical results were in agreement with those of the national standard method and ultraviolet spectrophotometry method, which indicated that the established method was reliable. CONCLUSIONSThe analytical method has the advantages of low reagent consumption, low cost and convenient operation, which can be used for the determination of inorganic and organic iodine contents. -
Key words:
- probe /
- rapid determination /
- organic iodine /
- inorganic iodine /
- edible salt /
- kelp
-

-
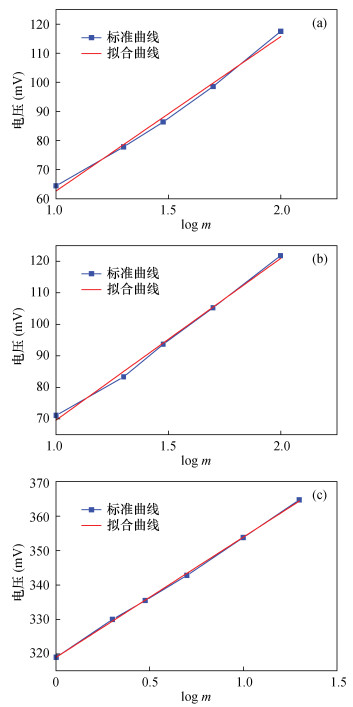
表 1 无机碘和有机碘标准溶液工作溶液中碘含量的相关测定数据
Table 1. Determination data of iodine content in iodine standard working solution
标准溶液浓度
(mg/L)食盐样品 标准溶液浓度
(mg/L)含碘物质样品 无机碘
(mV)有机碘
(mV)高浓度有机碘
(mV)0.1 58.90 - 1 318.92 0.2 64.39 71.11 2 329.90 0.4 77.82 83.32 3 335.40 0.6 86.37 93.69 5 342.72 1.0 98.58 105.29 10 353.71 2.0 117.50 121.79 20 364.70 表 2 食盐中无机碘离子含量测定结果
Table 2. Analytical results of inorganic iodine contents in eatable salt samples
样品 有机碘离子含量(mg/kg) RSD
(%)本法分次测定值 平均值 加碘精制盐 19.87 19.99 20.23 20.03 0.92 低钠盐 21.41 21.20 21.22 21.28 0.77 海藻加碘食盐 19.87 20.06 20.07 20.00 0.56 表 3 食盐和海带中有机碘离子含量测定结果
Table 3. Analytical results of organic iodine contents in eatable salt and kelp samples
样品 有机碘离子含量(mg/kg) RSD
(%)本法分次测定值 平均值 低钠盐 2.12 2.08 2.10 2.10 0.95 海藻加碘食盐 1.96 2.06 2.01 2.01 2.49 海带 2.4×103 2.9×103 3.4×103 2.9×103 - 表 4 食盐中无机碘离子含量标准加入测定结果
Table 4. Standard results of inorganic iodine contents in eatable salt samples
样品 无机碘离子含量(mg/kg) 加标后测定值
(mg/kg)回收率
(%)碘探针法 标准方法 紫外分光光度法 加碘精制盐 20.03 20.22 18.56 30.13 101.0 低钠盐 21.28 21.26 18.39 30.99 97.1 海藻加碘食盐 20.00 - 21.94 30.01 100.1 表 5 食盐中有机碘离子含量标准加入测定结果
Table 5. Standard results of organic iodine contents in eatable salt samples
样品 有机碘离子含量(mg/kg) 加标后测定值
(mg/kg)回收率
(%)碘探针法 标准方法 低钠盐 2.10 2.32 3.10 100.0 海藻加碘食盐 2.01 1.92 3.00 99.0 -
[1] Liu J F, Wang C G, Tang X L, et al.Correlation analysis of metabolic syndrome and its components with thyroid nodules[J].Targets and Therapy, 2019, 12:1617-1623. http://cn.bing.com/academic/profile?id=37bc019bb36e356dee50291a77adb675&encoded=0&v=paper_preview&mkt=zh-cn
[2] 王晓岑, 佟丽娟.通过对食用碘盐质量的检测分析防治碘缺乏病的措施探讨[J].中国医药指南, 2019, 17(1):295. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyyzn201901246
Wang X C, Tong L J.The measures to prevent and cure iodine deficiency disease were discussed by analyzing the quality of edible iodized salt[J].Chinese Medical Guide, 2019, 17(1):295. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyyzn201901246
[3] Chailapakul O, Amatatongchai M, Wilairat P, et al.Flow-injection determination of iodide ion in nuclear emergency tablets, using boron-doped diamond thin film electrode[J].Talanta, 2004, 64(5):1253-1258. doi: 10.1016/j.talanta.2004.04.023
[4] Cauduroa V H, Morgana D, Barin J S, et al.Successive digestions for pre-concentration and ultra-trace determination of Br and I by plasma-based atomic spectrometry and ion chromatography[J].Microchemical Journal, 2019, 147:239-244. doi: 10.1016/j.microc.2019.03.012
[5] Kim S Y, Park J M, Hwang J P.Analysis of iodine content in salts and Korean sauces for low-iodine diet education in Korean patients with thyroid cancer preparing for radioiodine therapy[J].Nuclear Medicine and Molecular Imaging, 2018, 52(3):229-233. doi: 10.1007/s13139-017-0511-8
[6] Xu L R, Zhu X F, Yu X Z, et al.Rapid and simultaneous determination of the iodine value and saponification number of edible oils by FTIR spectroscopy[J].Lipid Science and Technology, 2018, 120(4):1-20. http://cn.bing.com/academic/profile?id=fb6fa70c5c83a081c4887460c904cb66&encoded=0&v=paper_preview&mkt=zh-cn
[7] 金钦汉, 陈焕文, 曹彦波, 等.高灵敏度光度计[P].中国专利,
ZL 99254233.2. Jin Q H, Chen H W, Cao Y B, et al.Photometer of High Sensitivity[P].Chinese Patent, ZL 99254233.2.
[8] Ajenesh C, Matakite M, Surendra P.Determination of iodine content in Fijian foods using spectrophotometric kinetic method[J].Microchemical Journal, 2019, 148:475-479. doi: 10.1016/j.microc.2019.04.060
[9] Tavassoli-Kafrani M H, Voort F R, Curtis J M.The use of ATR-FTIR spectroscopy to measure changes in the oxirane content and iodine value of vegetable oils during epoxidation[J].Lipid Science and Technology, 2017, 119(7):1-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=95427e194f9c41f488199ef996585609
[10] Ryabukhinaa T S, Bazel Y R.Spectrophotometric determination of the total iodine content in drinking and mineral waters using the microextraction preconcentration[J].Journal of Water Chemistry and Technology, 2018, 40(4):228-233. doi: 10.3103/S1063455X18040082
[11] 连宁.容量法测定加碘盐中的碘[J].青海科技, 2000(1):29-30. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK200000904440
Lian N.Volumetric determination of iodine in iodized salt[J].Qinghai Science and Technology, 2000(1):29-30. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK200000904440
[12] Lu J P, Tan F W, Tang Q, et al.Novel method for indirect determination of iodine in marine products by atomic fluorescence spectrometry[J].Chemical Research in Chinese Universities, 2013, 29(1):26-29. doi: 10.1007/s40242-013-2171-2
[13] Cui W J, Hou H F, Chen J J, et al.The speciation analysis of iodate and iodide in high salt brine by high performance liquid chromatography and inductively coupled plasma mass spectrometry[J].Royal Society of Chemistry, 2019, 34:1374-1379. http://cn.bing.com/academic/profile?id=7c17ff06f39393a2ec3f328f22bca500&encoded=0&v=paper_preview&mkt=zh-cn
[14] 陈俊良, 杨红霞, 刘崴, 等.HPLC-ICP-MS法研究内蒙古锡盟和新疆塔城高碘地区地下水的总碘及碘形态特征[J].岩矿测试, 2017, 36(6):614-623. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201707040114
Chen J L, Yang H X, Liu W, et al.Study on the total iodine and iodine speciation characteristics in Xilingol League, Inner Mongolia and Tacheng, Xinjiang high iodine area by high performance liquid chromatography-inductively coupled plasma-mass spectrometry[J].Rock and Mineral Analysis, 2017, 36(6):614-623. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201707040114
[15] Vance K A, Makhmudov A, Shakirova G, et al.Determin-ation of iodine content in dairy products by inductively coupled plasma mass spectrometry[J].Atomic Spectroscopy, 2018, 39(3):95-99. https://www.sciencedirect.com/science/article/pii/S0026265X16306233
[16] 侯小琳, 冯向前, 李春生, 等.碘在生物样品消解和灰化过程中的丢失研究[J].核化学与放射化学, 1998, 20(4):242-246. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800266659
Hou X L, Feng X Q, Li C S, et al.Study on iodine loss in biological sample digestion and ashing[J].Jounal of Nuclear and Radiochemistry, 1998, 20(4):242-246. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800266659
[17] Christine D, Sarah M, Olivier G.Determination of iodine in polyamide by inductively-coupled plasma/mass spectrometry[J].Talanta, 2018, 189:568-572. doi: 10.1016/j.talanta.2018.07.054
[18] Gorbunova M O, Baulina A A, Kulyaginova M S, et al.Dynamic gas extraction of iodine in combination with a silver triangular nanoplate-modified paper strip for colorimetric determination of iodine and of iodine-interacting compounds[J].Microchimica Acta, 2019, 186(3):2-9. http://cn.bing.com/academic/profile?id=58a4de694ca4eabdba79603bcd2f9cf1&encoded=0&v=paper_preview&mkt=zh-cn
[19] 李洪伟, 刘晓端, 李保山.地下水和土壤中不同形态碘的分离测定[J].岩矿测试, 2009, 28(4):337-341. doi: 10.3969/j.issn.0254-5357.2009.04.007 http://www.ykcs.ac.cn/article/id/ykcs_20090407
Li H W, Liu X D, Li B S.Separation and determination of different iodine species in ground water and soil samples[J].Rock and Mineral Analysis, 2009, 28(4):337-341. doi: 10.3969/j.issn.0254-5357.2009.04.007 http://www.ykcs.ac.cn/article/id/ykcs_20090407
[20] 李艳红.离子选择性电极在化工分析领域中的应用[J].云南化工, 2018, 45(6):49-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ynhg201806022
Li Y H.Application of ion selective electrode in chemical analysis[J].Yunnan Chemical Industry, 2008, 45(6):49-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ynhg201806022
[21] 王鹏, 张国联.含电解质的干式离子选择电极的內建[J].化学与生物工程, 2019, 36(8):59-61. doi: 10.3969/j.issn.1672-5425.2019.08.012
Wang P, Zhang G L.Construction of dry ion selective electrode containing electrolyte[J].Chemistry & Bioengineering, 2019, 36(8):59-61. doi: 10.3969/j.issn.1672-5425.2019.08.012
[22] 代鸿章, 王登红, 刘丽君, 等.电子探针和微区X射线衍射研究陕西镇安钨-铍多金属矿床中祖母绿级绿柱石[J].岩矿测试, 2018, 37(3):336-345. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201712140193
Dai H Z, Wang D H, Liu L J, et al.Study on emerald-level beryl from the Zhen'an W-Be polymetallic deposit in Shaanxi Province by electron probe microanalyzer and micro X-ray diffractometer[J].Rock and Mineral Analysis, 2018, 37(3):336-345. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201712140193
[23] Li J X, Wang Y Y, Qian Y, et al.Iodine in ground-water of the North China Plain:Spatial patterns and hydrogeochemical processes of enrichment[J].Journal of Geochemical Exploration, 2013, 135:40-53. doi: 10.1016/j.gexplo.2012.11.016
[24] Small L J, Krumhansl J L, Rademacher D X, et al.Iodine Detection in Ag-mordenite Based Sensors:Charge Conduction Pathway Determinations[M].Microporous and Mesoporous Materials, 2019.
[25] de O.Costa G, Feiteira F N, de M.Schuenck H, et al.Iodine determination in table salts by digital images analysis[J].Analytical Methods, 2018, 10:4463-4470. doi: 10.1039/C8AY01248B
[26] 刘烨, 杨丽梅, 张磊, 等.海藻食盐碘含量测定新方法研究[J].中国井矿盐, 2017, 48(2):31-33. doi: 10.3969/j.issn.1001-0335.2017.02.012
Liu Y, Yang L M, Zhang L, et al.A new method for the determination of iodine in algal salt[J].China Mine Salt, 2017, 48(2):31-33. doi: 10.3969/j.issn.1001-0335.2017.02.012
[27] 周贤亚.海带中碘含量的测定[J].广东化工, 2018, 45(13):276. http://d.old.wanfangdata.com.cn/Periodical/sdhg201308032
Zhou X Y.Determination of iodine content in kelp[J].Guangdong Chemical Industry, 2008, 45(13):276. http://d.old.wanfangdata.com.cn/Periodical/sdhg201308032
[28] 陈翔.利用Execl图表功能绘制离子选择电极法标准曲线[J].中国卫生检验杂志, 2002, 12(2):236-237. doi: 10.3969/j.issn.1004-8685.2002.02.076
Chen X.The standard curve of ion selective electrode method is drawn by using the chart function of Execl[J].Chinese Journal of Health Laboratory Technology, 2002, 12(2):236-237. doi: 10.3969/j.issn.1004-8685.2002.02.076
[29] Aydin I, Temel Z, Gunduz B, et al.Comparative deter-mination of phosphorus fractions in coastal surface sediment (NE Mediterranean Sea) by ICP-OES and UV/Vis spectrometry[J].Atomic Spectroscopy, 2018, 39(5):193-197.
[30] 宋树成, 郭如侠.浅谈样品加标回收率[J].水科学与工程技术, 2011, 11(4):92-93. doi: 10.3969/j.issn.1672-9900.2011.04.036
Song S C, Guo R X.A brief discussion on the standard recovery rate of samples[J].Water Sciences and Engineering Technology, 2011, 11(4):92-93. doi: 10.3969/j.issn.1672-9900.2011.04.036
-



 下载:
下载: