Determination of the Redox Potential for Aqueous Fe(Ⅱ)-Goethite Heterogeneous Systems by Potentiometric Method
-
摘要:
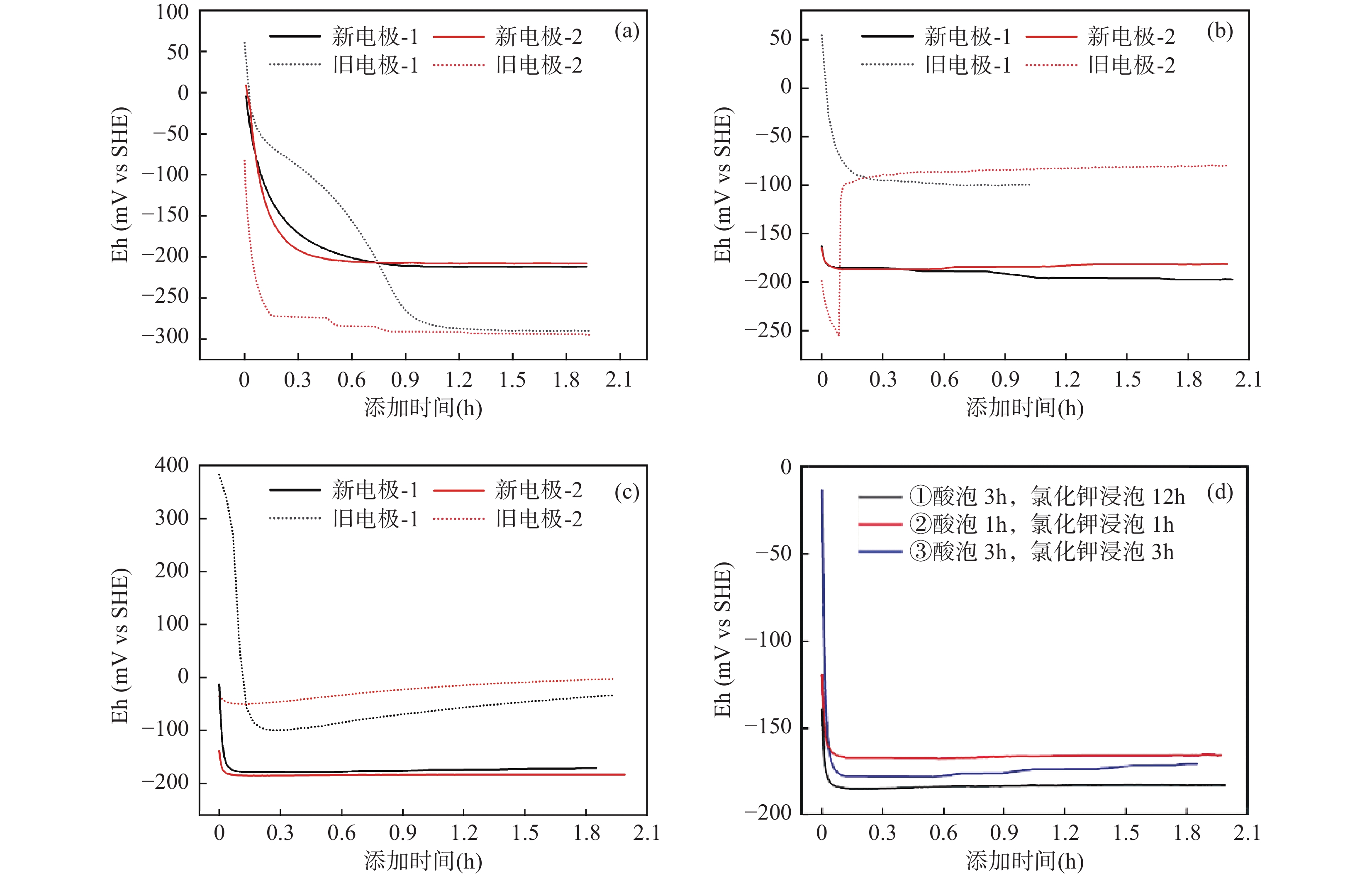
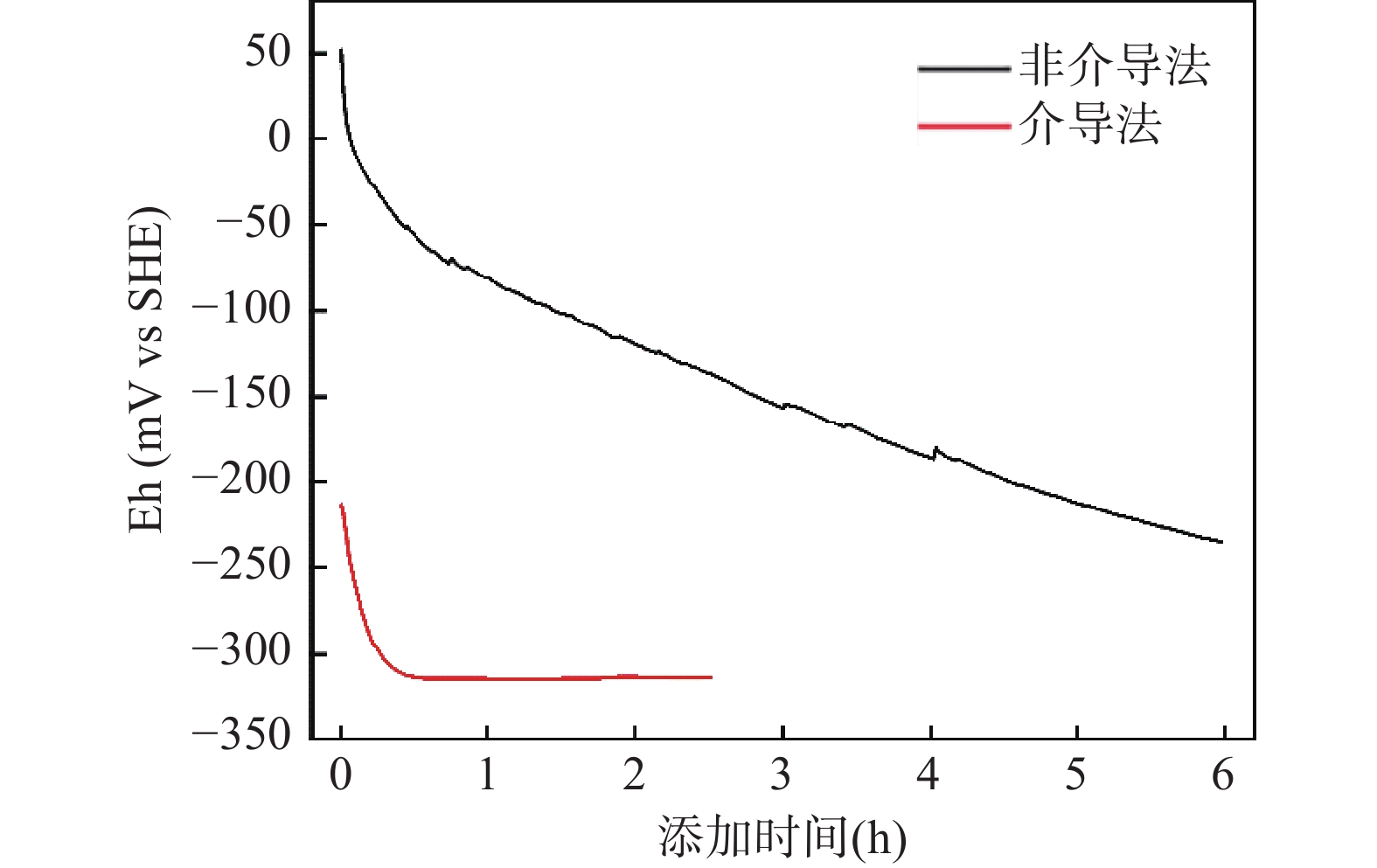
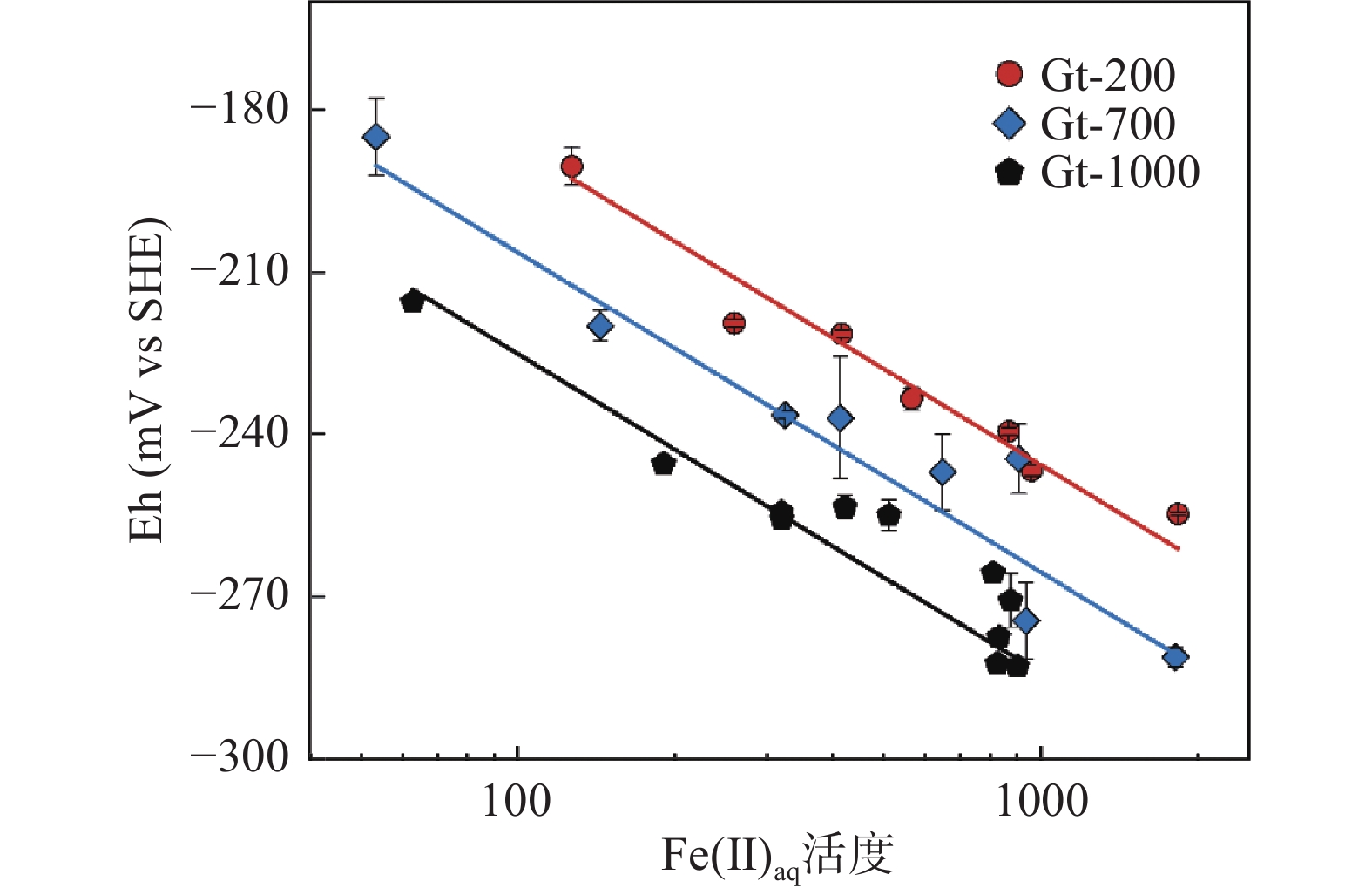
溶解性二价铁(Feaq 2+)-铁氧化物非均相体系的氧化还原能力显著影响含水层中有毒重金属和有机污染物的迁移转化行为。然而,非均相体系氧化还原电位(Eh)的测定存在平衡缓慢和结果不稳定等难题,故亟待开发与优化电化学法快速准确测定Eh,实现Feaq 2+-铁氧化物非均相体系氧化还原能力的定量表征。本文选取针铁矿作为常见的铁氧化物,探讨了介导和非介导电位法测定Feaq 2+-针铁矿非均相体系Eh的优化实验条件,研究了介导物质的类型与添加顺序以及工作电极预处理方法等条件对Eh测定的影响,并通过能斯特方程验证电位法的可行性。结果表明:工作电极的表面状态、介导物质的选择以及针铁矿-工作电极之间平衡的建立对Eh 测定十分关键。介导法能有效地缩短测定时间,但需要选择合适的介导物质;非介导法需确保工作电极与针铁矿之间平衡的建立。同时,利用非介导法测定了不同pH和Feaq 2+浓度下不同粒径针铁矿与Feaq 2+非均相体系的Eh,根据能斯特方程和最小二乘法多元线性拟合得到不同粒径针铁矿的标准氧化还原电位(Eh0),对于粒径为200、700和1000nm的针铁矿Eh0分别为815、802和782mV,验证了优化后电位法的可行性。本文的结论可为矿物非均相体系Eh的测定提供方法学参考,也为预测Feaq 2+-铁氧化物非均相体系参与的污染物衰减速率提供理论支撑。
Abstract:The redox properties of Feaq 2+-iron oxide heterogeneous systems play an important role in the environmental behaviors of heavy metal and organic pollutants. However, the determination of Eh for heterogeneous systems is still challenging due to the redox equilibration between the electrode and suspension is sluggish. In this study, goethite was chosen as the representative of iron oxide in the aquifer. Both mediated and non-mediated potential methods were conducted to obtain the Eh of Feaq 2+-goethite heterogeneous system through the optimizing of measurement conditions. Moreover, the effect of the working electrode surface state, the kind of mediators, and the equilibrium time on the Eh measurement were investigated. The results indicated that the working electrode surface state, the suitable mediators, and the establishment of equilibrium were crucial for obtaining accurate Eh. Compared with the non-mediated potentiometric method, the mediated method can shorten the determination time, but the appropriate mediator must be chosen. In non-mediated potentiometric method, the equilibrium between the working electrode and goethite must be established. The standard redox potential (Eh0) of goethite with different particle sizes was obtained, which demonstrated the practicability of this method. The results provide the method for the Eh measurement of mineral heterogeneous systems, and also provide theoretical support for predicting the pollutant abiotic attenuation rate induced by Feaq 2+-iron oxide heterogeneous systems.
-
Key words:
- dissolved bivalent iron /
- iron oxide /
- heterogeneous system /
- redox potential /
- goethite
-

-
表 1 不同pH和Feaq 2+浓度下应用介导电位法与非介导电位法测定Eh的对比
Table 1. Comparison of Eh values obtained by mediated and non-mediated potentiometric method under different pH and Feaq 2+ concentrations
实验条件 非介导法
Eh测定值
(mV)介导法Eh测定值
(mV)Eh理论值
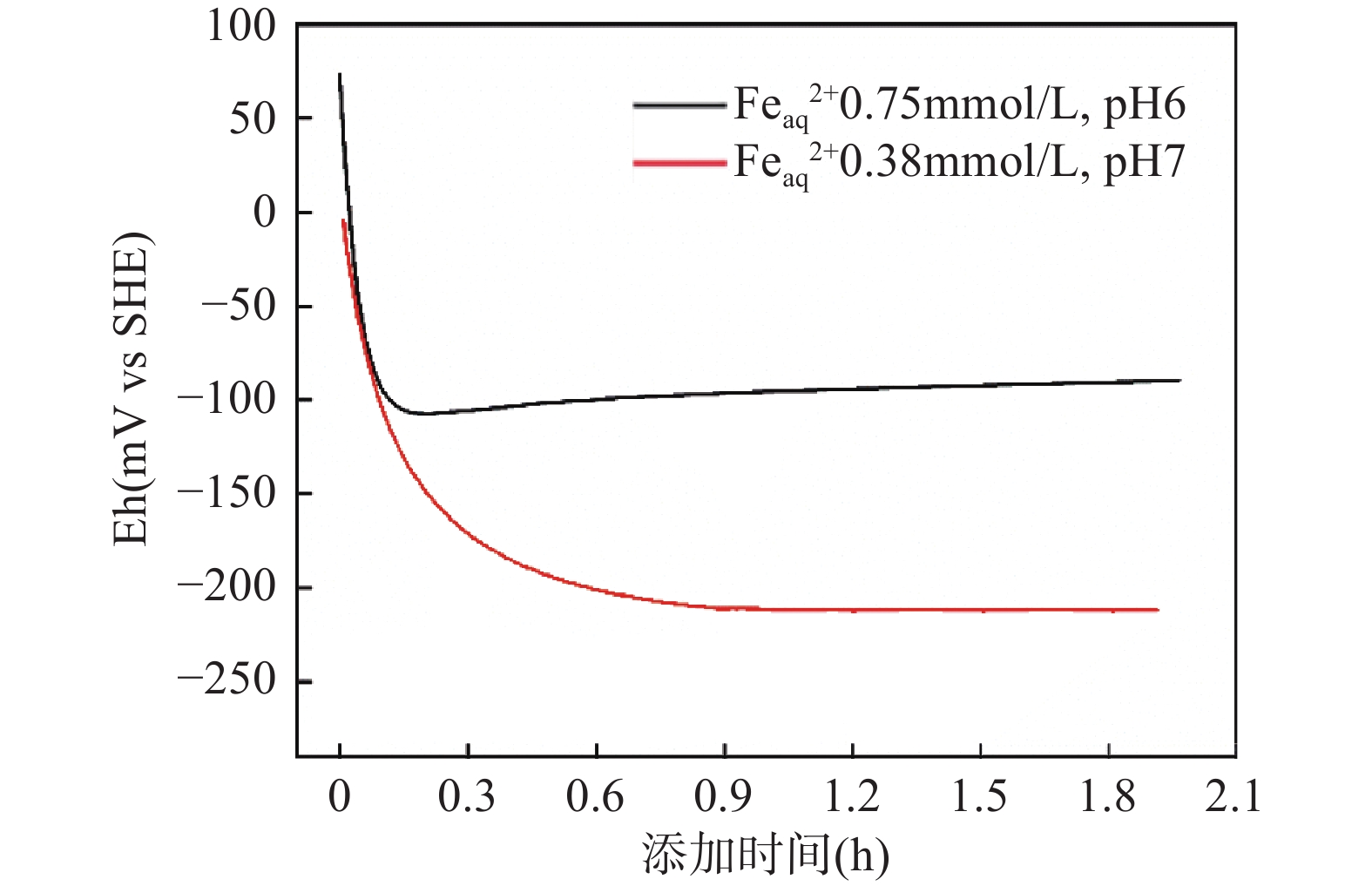
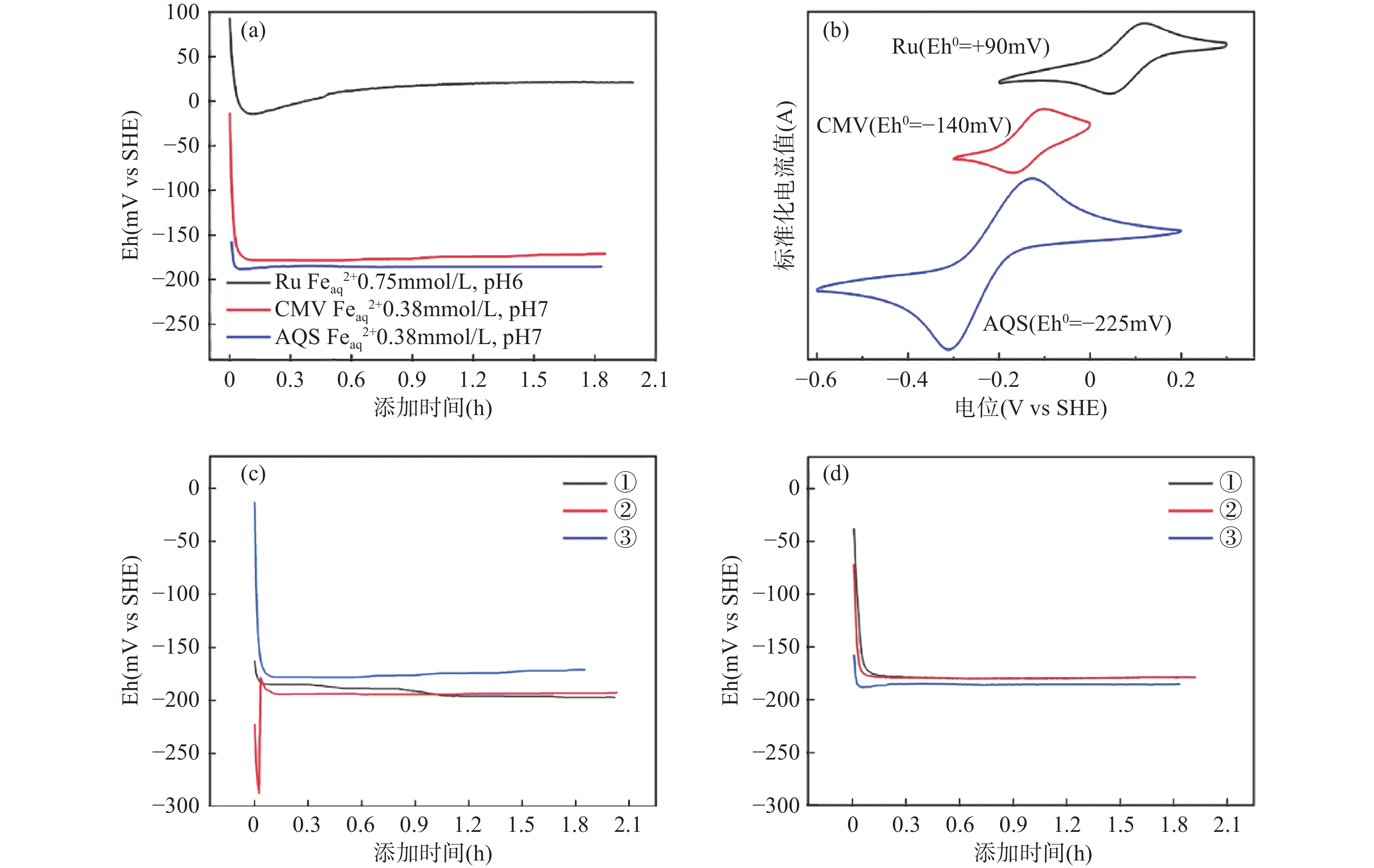
(Eh0=804mV)差异(mV) Ru CMV AQS 非介导法 Ru CMV AQS Feaq 2+ 0.75mmol/L,pH 6 −90 +21 / / −56 −34 77 / / Feaq 2+ 0.38mmol/L,pH 7 −211 / −176 −185 −215 +4 / +39 +30 注:“/”表示未取得实验测定值。 -
[1] 郭华明, 高志鹏, 修伟. 地下水典型氧化还原敏感组分迁移转化的研究热点和趋势[J]. 地学前缘, 2022, 29(3): 64−75. doi: 10.13745/j.esf.sf.2022.1.37
Guo H M, Gao Z P, Xiu W. Typical redox-sensitive components in groundwater systems: Research highlights and trends[J]. Earth Science Frontiers, 2022, 29(3): 64−75. doi: 10.13745/j.esf.sf.2022.1.37
[2] Kappler A, Bryce C, Mansor M, et al. An evolving view on biogeochemical cycling of iron[J]. Nature Reviews Microbiology, 2021, 19(6): 360−374. doi: 10.1038/s41579-020-00502-7
[3] Coyte R M, Vengosh A. Factors controlling the risks of co-occurrence of the redox-sensitive elements of arsenic, chromium, vanadium, and uranium in groundwater from the Eastern United States[J]. Environmental Science & Technology, 2020, 54(7): 4367−4375. doi: 10.1021/acs.est.9b06471
[4] Borch T, Campbell K, Kretzschmar R, et al. How electron flow controls contaminant dynamics[J]. Environmental Science & Technology, 2010, 44(1): 3−6. doi: 10.1021/es903264z
[5] 姚远, 余光辉, 滕辉. 土壤铁氧化物-亚铁的相互作用及其环境影响研究进展[J]. 土壤, 2023, 55(4): 718−728. doi: 10.13758/j.cnki.tr.2023.04.004
Yao Y, Yu G H, Teng H. Soil iron oxide-ferrous interaction and its environmental effects: A review[J]. Soils, 2023, 55(4): 718−728. doi: 10.13758/j.cnki.tr.2023.04.004
[6] 胡敏, 李芳柏, 土壤微生物铁循环及其环境意义[J]. 土壤学报, 2014, 51(4): 683−698.
Hu M, Li F B. Soil microbe mediated iron cycling and its environmental implication[J]. Acta Pedologica Sinica, 2014, 51(4): 683−698.
[7] Kappler A, Schink B, Newman D K. Fe(Ⅲ) mineral formation and cell encrustation by the nitrate-dependent Fe(Ⅱ)-oxidizer strain bofen1[J]. Geobiology, 2005, 3(4): 235−245. doi: 10.1111/j.1472-4669.2006.00056.x
[8] 罗伟嘉, 冯晨, 侯国华, 等. 铁氧化物-二价铁体系在潜流带低渗透区的氧化还原特性研究进展[J]. 岩矿测试, 2024, 43(2): 375−396. doi: 10.15898/j.ykcs.202309090150
Luo W J, Feng C, Hou G H, et al. Progress on redox characteristics of an iron oxide-ferrous system in the hyporheic zone[J]. Rock and Mineral Analysis, 2024, 43(2): 375−396. doi: 10.15898/j.ykcs.202309090150
[9] 谷春云, 廖高明, 邓一荣, 等. FeS介导下的1, 2-二溴乙烷非生物自然衰减[J]. 中国环境科学, 2023, 43(9): 4632−4638. doi: 10.19674/j.cnki.issn1000-6923.20230523.009
Gu C Y, Liao G M, Deng Y R, et al. The abiotic natural attenuation of 1, 2-dibromoethane mediated by FeS[J]. China Environmental Science, 2023, 43(9): 4632−4638. doi: 10.19674/j.cnki.issn1000-6923.20230523.009
[10] 廖高明, 马杰, 谷春云, 等. 污染场地卤代烃非生物自然衰减研究进展[J]. 环境科学研究, 2021, 34(3): 742−754. doi: 10.13198/j.issn.1001-6929.2020.09.14
Liao G M, Ma J, Gu C Y, et al. Research progress on abiotic natural attenuation of halogenated hydrocarbons at contaminated sites[J]. Research of Environmental Sciences, 2021, 34(3): 742−754. doi: 10.13198/j.issn.1001-6929.2020.09.14
[11] Cardenas-Hernandez P A, Anderson K A, Murillo-Gelvez J, et al. Reduction of 3-nitro-1, 2, 4-triazol-5-one (NTO) by the hematite-aqueous Fe(Ⅱ) redox couple[J]. Environmental Science & Technology, 2020, 54(19): 12191−12201. doi: 10.1021/acs.est.0c03872
[12] 王娜, 王家松, 曾江萍, 等. 重铬酸钾和高锰酸钾电位落差法测定砂岩型铀矿氧化还原电位的探讨[J]. 岩矿测试, 2022, 41(5): 806−814. doi: 10.15898/j.cnki.11-2131/td.202112080199
Wang N, Wang J S, Zeng J P, et al. Determination of redox potential of sandstone-type uranium ore by potential drop methods of potassium dichromate and potassium permanganate[J]. Rock and Mineral Analysis, 2022, 41(5): 806−814. doi: 10.15898/j.cnki.11-2131/td.202112080199
[13] Neumann A, Hofstetter T B, Luessi M, et al. Assessing the redox reactivity of structural iron in smectites using nitroaromatic compounds as kinetic probes[J]. Environmental Science & Technology, 2008, 42(22): 8381−8387. doi: 10.1021/es801840x
[14] Aeschbacher M, Sander M, Schwarzenbach R P. Novel electrochemical approach to assess the redox properties of humic substances[J]. Environmental Science & Technology, 2010, 44(1): 87−93. doi: 10.1021/es902627p
[15] Sander M, Hofstetter T B, Gorski C A. Electrochemical analyses of redox-active iron minerals: A review of nonmediated and mediated approaches[J]. Environmental Science & Technology, 2015, 49(10): 5862−5878. doi: 10.1021/acs.est.5b00006
[16] Aeppli M, Kaegi R, Kretzschmar R, et al. Electrochemical analysis of changes in iron oxide reducibility during abiotic ferrihydrite transformation into goethite and magnetite[J]. Environmental Science & Technology, 2019, 53(7): 3568−3578. doi: 10.1021/acs.est.8b07190
[17] 陈袁波, 邓思宇, 余珂, 等. 泥炭沼泽湿地土壤分解过程中可溶性有机质氧化还原能力变化特征及其影响机制[J]. 生态学报, 2020, 40(24): 8948−8957.
Chen Y B, Deng S Y, Yu K, et al. Change and influence mechanism of the dissolved organic matter redox capacity during peat bog soil decomposition[J]. Acta Ecologica Sinica, 2020, 40(24): 8948−8957.
[18] Kocur C M D, Fan D, Tratnyek P G, et al. Predicting abiotic reduction rates using cryogenically collected soil cores and mediated reduction potential measurements[J]. Environmental Science & Technology Letters, 2019, 7(1): 20−26. doi: 10.1021/acs.estlett.9b00665
[19] Gorski C A, Edwards R, Sander M, et al. Thermo-dynamic characterization of iron oxide-aqueous Fe2+ redox couples[J]. Environmental Science & Technology, 2016, 50(16): 8538−8547. doi: 10.1021/acs.est.6b02661
[20] Shi Z, Nurmi J T, Tratnyek P G. Effects of nano zero-valent iron on oxidation-reduction potential[J]. Environmental Science & Technology, 2011, 45(4): 1586−1592. doi: 10.1021/es103185t
[21] Aeppli M, Voegelin A, Gorski C A, et al. Mediated electrochemical reduction of iron (oxyhydr-) oxides under defined thermodynamic boundary conditions[J]. Environmental Science & Technology, 2018, 52(2): 560−570. doi: 10.1021/acs.est.7b04411
[22] 李欣, 杨珊珊, 刘菲, 等. 典型铁氧化物-Fe(Ⅱ)aq非均相体系氧化还原电位的测定方法[J]. 分析化学, 2023, 51(12): 1935−1944. doi: 10.19756/j.issn.0253-3820.231037
Li X, Yang S S, Liu F, et al. Detection of redox potential of typical iron oxide-Fe(Ⅱ)aq heterogeneous system[J]. Chinese Journal of Analytical Chemistry, 2023, 51(12): 1935−1944. doi: 10.19756/j.issn.0253-3820.231037
[23] Robinson T C, Latta D E, Leddy J, et al. Redox potentials of magnetite suspensions under reducing conditions[J]. Environmental Science & Technology, 2022, 56(23): 17454−17461. doi: 10.1021/acs.est.2c05196
[24] Wang D, Crowe W E, Strongin R M, et al. Exploring the pH dependence of viologen reduction by α-carbon radicals derived from Hcy and Cys[J]. Chemical Communications, 2009(14): 1876−1878. doi: 10.1039/b819746f
[25] Villacis-Garcia M, Ugalde-Arzate M, Vaca-Escobar K, et al. Laboratory synthesis of goethite and ferrihydrite of controlled particle sizes[J]. Boletin De La Sociedad Geologica Mexicana, 2015, 67(3): 433−446. doi: 10.18268/BSGM2015v67n3a7
[26] Tamura H, Goto K, Yotsuyanagi T, et al. Spectrophotometric determination of iron(Ⅱ) with 1, 10-phenanthroline in the presence of large amounts of iron(Ⅲ)[J]. Talanta, 1974, 21(4): 314−318. doi: 10.1016/0039-9140(74)80012-3
[27] Fultz M L, Durst R A. Mediator compounds for the electrochemical study of biological redox systems a compilation[J]. Analytica Chimica Acta, 1982, 140(1): 1−18. doi: 10.1016/s0003-2670(01)95447-9
[28] Roden E E. Geochemical and microbiological controls on dissimilatory iron reduction[J]. Comptes Rendus Geoscience, 2006, 338(6−7): 456−467. doi: 10.1016/j.crte.2006.04.009
[29] Teasdale P R, Minett A I, Dixon K, et al. Practical improvements for redox potential (Eh) measurements and the application of a multiple-electrode redox probe (MERP) for characterizing sediment in situ[J]. Analytica Chimica Acta, 1998, 367(1-3): 201−213. doi: 10.1016/s0003-2670(98)00171-8
[30] Chen G, Thompson A, Gorski C A. Disentangling the size-dependent redox reactivity of iron oxides using thermodynamic relationships[J]. Proceedings of the National Academy of Sciences, 2022, 119(40): 1−10. doi: 10.1073/pnas.2204673119
[31] Stewart S M, Hofstetter T B, Joshi P, et al. Linking thermodynamics to pollutant reduction kinetics by Fe2+ bound to iron oxides[J]. Environmental Science & Technology, 2018, 52(10): 5600−5609. doi: 10.1021/acs.est.8b00481
-



 下载:
下载: