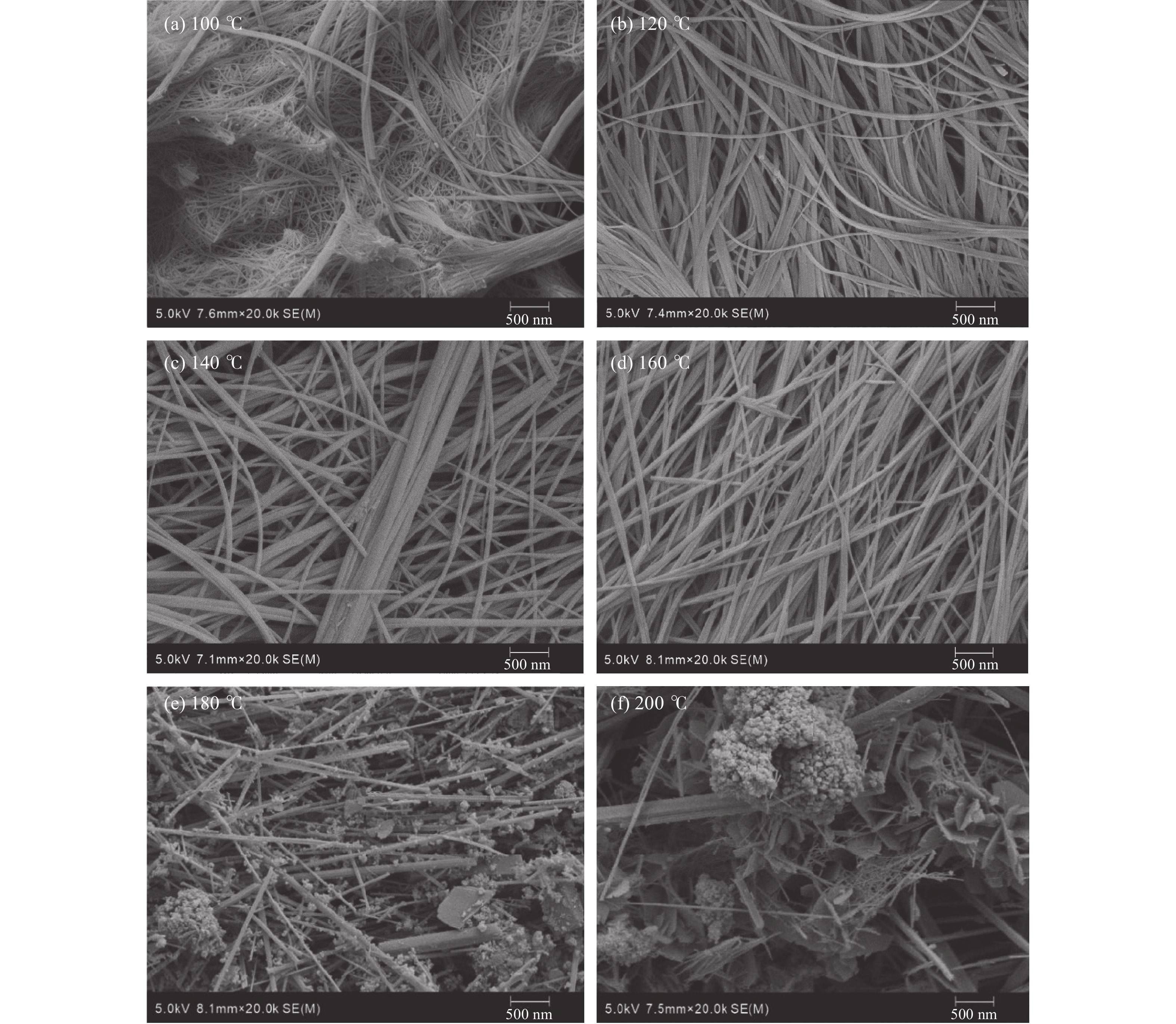
Carbon sequestration research of synthesizing dawsonite using CO2 under different experimental conditions
-
摘要:
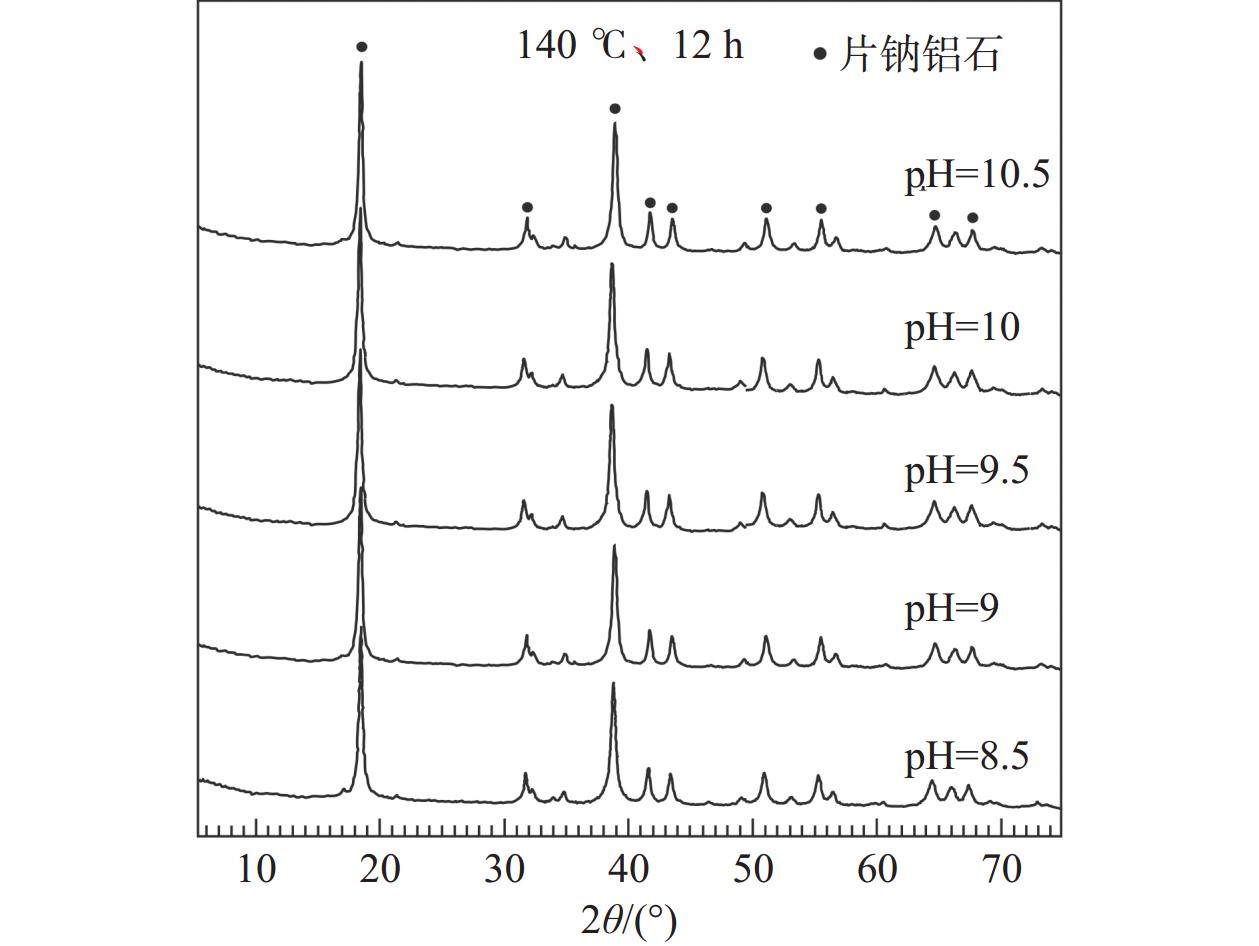
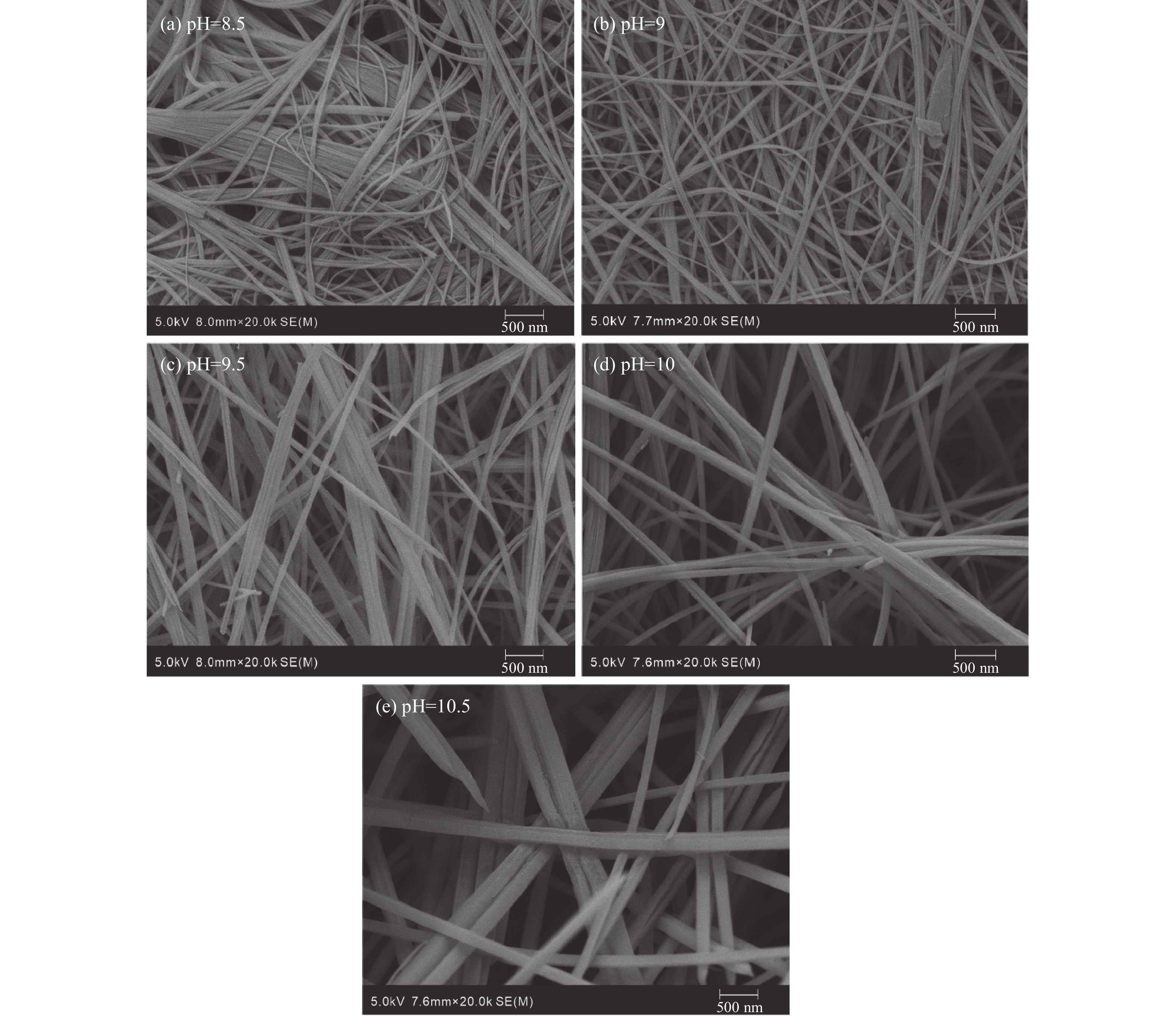
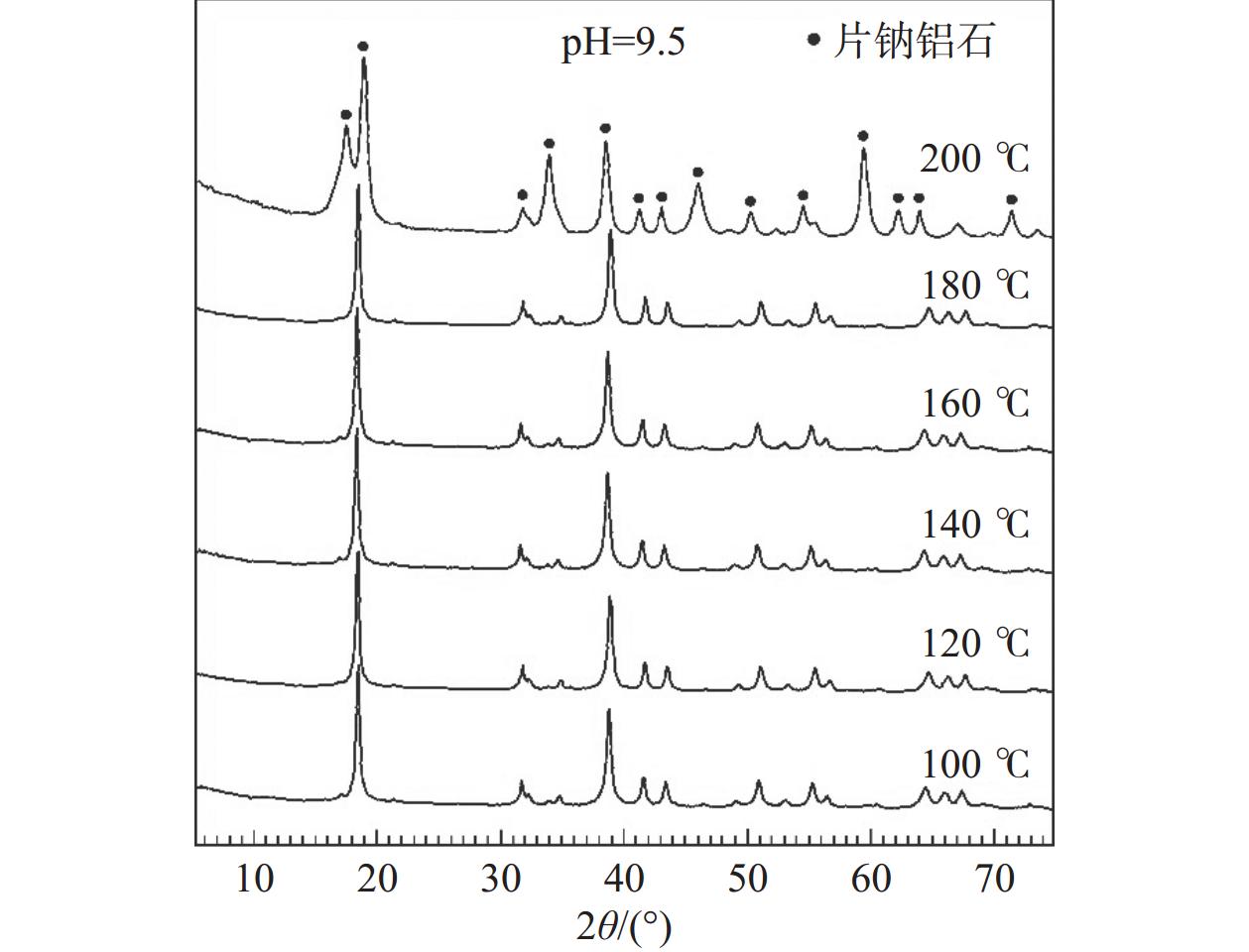
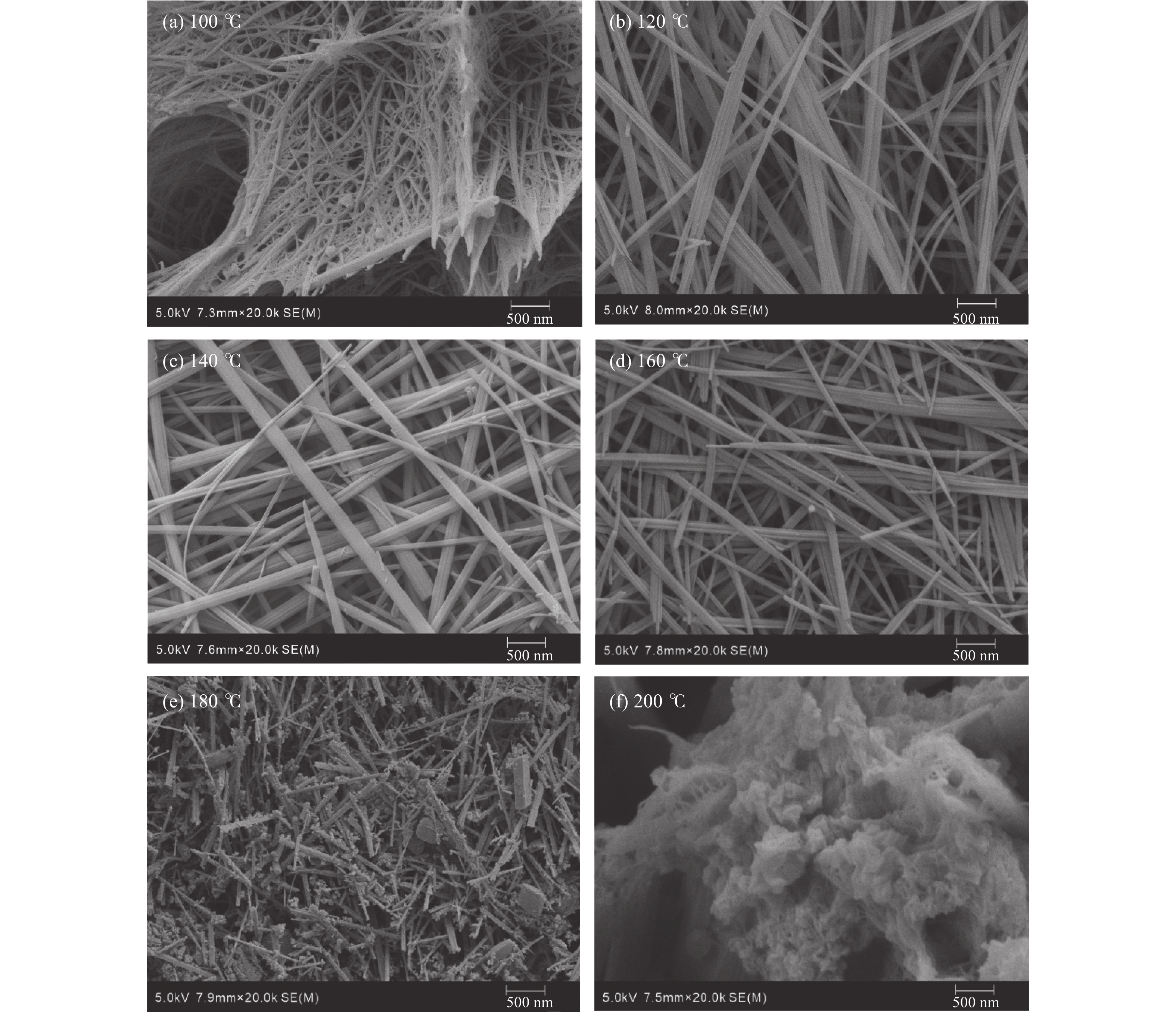
CO2的矿物捕获是最持久、最稳定的地质封存形式。片钠铝石作为一种天然的CO2示踪矿物,其形成与CO2注入密切相关,也是CO2地质封存的重要固碳矿物。片钠铝石在地质背景中大量稳定存在的条件是制约CO2矿化捕获的关键问题,也是寻找CO2地质埋藏地点的重要影响因素。为了探索利用CO2快速合成片钠铝石的条件,对pH(8.5/9/9.5/10/10.5)、温度(100/120/140/160/180/200 ℃)和反应时间(6/12 h)3个主要影响因素进行了比较实验,并基于扫描电镜、X射线衍射分析阐明了合成片钠铝石的最佳条件。实验表明:在pH 为8.5~10.5和温度为100~180 ℃的范围内,产物均为纯片钠铝石,合成量随pH和温度的升高呈先增加后减少的趋势,在200 ℃的温度下,片钠铝岩的结晶度降低,拟薄水铝石的含量增加。延长反应时间对产品质量没有明显的促进作用,在200 ℃下延长反应时间反而会加速片钠铝石的溶解。总的来说,140 ℃和pH 9.5是CO2合成片钠铝石的最佳条件,也可能是地质封存CO2的理想条件。
Abstract:Mineral trapping of CO2 is the most durable and stable form of geological storage. As a natural CO2 tracer mineral, the formation of dawsonite is closely related to CO2 infusion, and it also be an important carbon fixation mineral for CO2 geological storage. The condition of massive and stable presence of dawsonite in geological background is a key issue that constrains the CO2 mineralization capture, and is also an important influencing factor in the search for CO2 geological burial sites. To explore the conditions for the rapid synthesis of dawsonite with CO2, we conducted comparative experiments for three main influencing factors of temperature (100/120/140/160/180/200 ℃), pH (8.5/9/9.5/10/10.5), and reaction time (6/12 h). Based on scanning electron microscopy, X-ray diffraction analysis was conducted to clarify the optimal conditions for the synthesis of dawsonite. The experiments suggested that in the range of pH 8.5–10.5 and temperature 100–180 ℃, the products were all pure dawsonite, and the synthesis amount showed a trend of increasing and then decreasing with the increase of pH and temperature. At 200 ℃, the crystallinity of dawsonite decreased and the content of pseudo boehmite increased. The prolongation of reaction time did not have an obvious promotion effect on the quality of the products, and the prolongation of reaction time at 200 ℃ would accelerate the dissolution of dawsonite instead. Overall, 140 ℃ and pH 9.5 are the best conditions for the synthesis of dawsonite from carbon dioxide and probably the ideal conditions for geological sequestration of carbon dioxide.
-

-
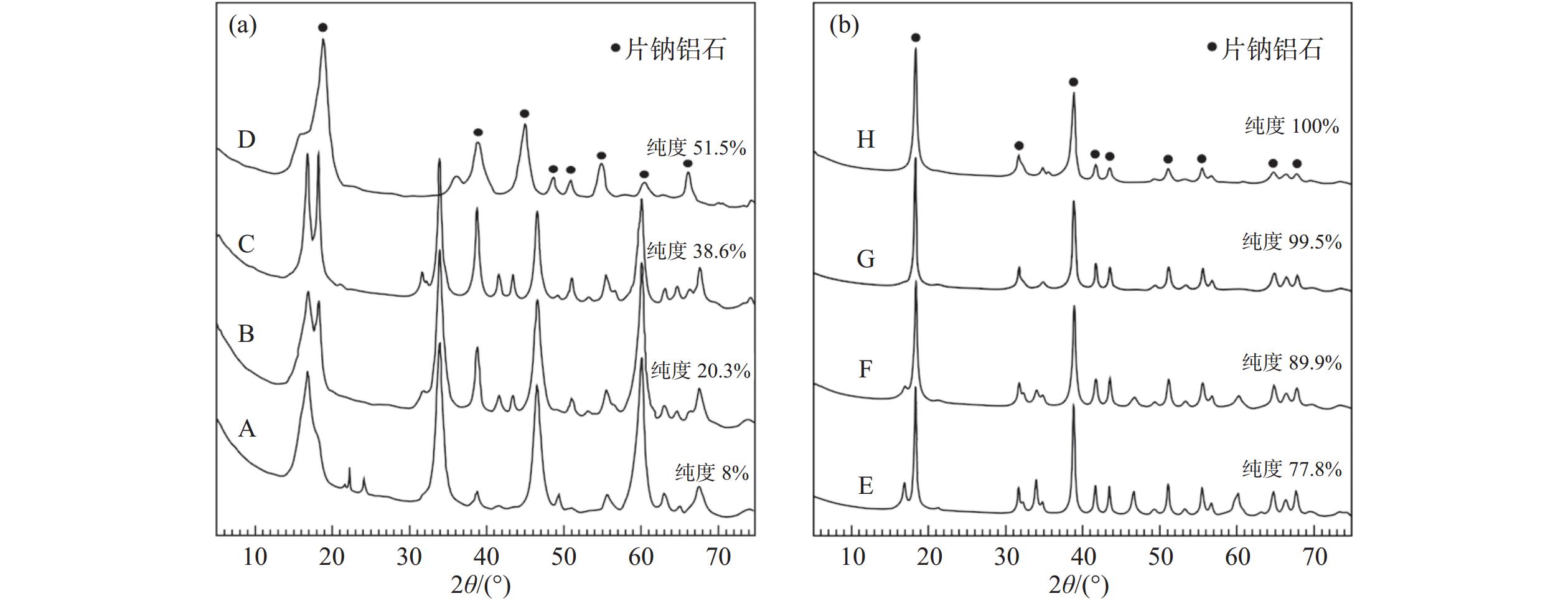
表 1 NaCl对片钠铝石产物纯度的影响对比实验结果
Table 1. Comparative experimental results of the effect of NaCl on dawsonite purity
序号 温度/℃ pH 是否加入 NaCl 片钠铝石的纯度/% 1 160 8.5 否 29.5 2 160 8.5 是 51.5 3 160 9.5 否 53.6 4 160 9.5 是 77.8 表 2 不同温度和pH值条件下反应12 h制得片钠铝石的质量
Table 2. Mass (g) of dawsonite prepared by 12 h reaction under different temperature and pH conditions
pH值 片钠铝石的质量/g 100 ℃ 120 ℃ 140 ℃ 160 ℃ 180 ℃ 200 ℃ 8.5 1.22 1.25 1.25 1.19 1.01 0.3 9 1.35 1.45 1.48 1.39 1.19 0.4 9.5 1.43 1.57 1.61 1.49 1.21 0.4 10 1.31 1.36 1.38 1.38 0.96 0.35 10.5 0.97 1.08 1.08 1.0 0.56 - 注:“-”表示没有制得片钠铝石。 表 3 不同实验条件下片钠铝石对CO2的固化率
Table 3. Solidification rate of dawsonite on CO2 under different experimental conditions
pH值 CO2固化率/% 100 ℃ 120 ℃ 140 ℃ 160 ℃ 180 ℃ 200 ℃ 8.5 7.97 8.51 8.84 8.67 7.49 2.51 9 8.81 9.82 10.20 10.07 9.18 2.97 9.5 9.22 10.51 11.33 10.77 9.18 2.97 10 8.60 9.17 9.76 10.10 7.25 2.72 10.5 6.30 7.21 7.48 7.26 4.11 - 注:“-”表示CO2固化率趋近于0。 -
[1] DANIELS J. Accelerating CCS 2023-2027:five years plan[R]. Canberra:Global Carbon Capture and Storage Institute,2022.
[2] ZERAI B,SAYLOR B Z,MATISOFF G. Computer simulation of CO2 trapped through mineral precipitation in the Rose Run Sandstone,Ohio[J]. Applied Geochemistry,2006,21(2):223-240. doi: 10.1016/j.apgeochem.2005.11.002
[3] BENSON S M,COLE D R. CO2 sequestration in deep sedimentary formations[J]. Elements,2008,4(5):325-331. doi: 10.2113/gselements.4.5.325
[4] Intergovernmental Panel on Climate Change Global warming of 1.5 ℃[M]. Cambridge:Cambridge University Press,2022.
[5] QIU Y,LAMERS P,DAIOGLOU V,et al. Environmental trade-offs of direct air capture technologies in climate change mitigation toward 2100[J]. Nature Communications,2022,13(1):3635. doi: 10.1038/s41467-022-31146-1
[6] SHU D Y,DEUTZ S,WINTER B A,et al. The role of carbon capture and storage to achieve net-zero energy systems:trade-offs between economics and the environment[J]. Renewable and Sustainable Energy Reviews,2023,178:113246. doi: 10.1016/j.rser.2023.113246
[7] International Energy Agency. Zero by 2050:a roadmap for global energy sector[R]. Paris:International Energy Agency,2011.
[8] LIU S Q,LIU T,ZHENG S J,et al. Evaluation of carbon dioxide geological sequestration potential in coal mining area[J]. International Journal of Greenhouse Gas Control,2023,122:103814. doi: 10.1016/j.ijggc.2022.103814
[9] LOHUIS J O. Carbon dioxide disposal and sustainable development in the Netherlands[J]. Energy Conversion and Management,1993,34(9/11):815-821. doi: 10.1016/0196-8904(93)90024-5
[10] BACHU S,GUNTER W D,PERKINS E H. Aquifer disposal of CO2:hydrodynamic and mineral trapping[J]. Energy Conversion and Management,1994,35(4):269-279. doi: 10.1016/0196-8904(94)90060-4
[11] WARD C R. Analysis and significance of mineral matter in coal seams[J]. International Journal of Coal Geology,2002,50(1/4):135-168.
[12] ALCALDE J,FLUDE S,WILKINSON M,et al. Estimating geological CO2 storage security to deliver on climate mitigation[J]. Nature Communications,2018,9(1):2201. doi: 10.1038/s41467-018-04423-1
[13] LANE J,GREIG C,GARNETT A. Uncertain storage prospects create a conundrum for carbon capture and storage ambitions[J]. Nature Climate Change,2021,11(11):925-936. doi: 10.1038/s41558-021-01175-7
[14] ALSHAMMARI A,LAKSHMI V,BRANTLEY D,et al. Simulation of carbon dioxide mineralization and its effect on fault leakage rates in the South Georgia rift basin,southeastern US[J]. Heliyon,2022,8(6):e09635. doi: 10.1016/j.heliyon.2022.e09635
[15] HEPBURN C,ADLEN E,BEDDINGTON J,et al. The technological and economic prospects for CO2 utilization and removal[J]. Nature,2019,575(7781):87-97. doi: 10.1038/s41586-019-1681-6
[16] SNÆBJÖRNSDÓTTIR S Ó,SIGFÚSSON B,MARIENI C,et al. Carbon dioxide storage through mineral carbonation[J]. Nature Reviews Earth & Environment,2020,1(2):90-102.
[17] MADHAV D,COPPITTERS T,JI Y,et al. Amino acid promoted single-step carbon dioxide capture and mineralization integrated with polymer-mediated crystallization of carbonates[J]. Journal of Cleaner Production,2023,415:137845. doi: 10.1016/j.jclepro.2023.137845
[18] ZHANG G R,LU P,HUANG Y,et al. Investigation of mineral trapping processes based on coherent front propagation theory:a dawsonite-rich natural CO2 reservoir as an example[J]. International Journal of Greenhouse Gas Control,2021,110:103400. doi: 10.1016/j.ijggc.2021.103400
[19] XU T,APPS J A,PRUESS K. Numerical simulation to study mineral trapping for CO2 disposal in deep aquifer[J]. Applied Geochemistry,2004,19(6):917-936. doi: 10.1016/j.apgeochem.2003.11.003
[20] XU T,APPS J A,PRUESS K. Mineral sequestration of carbon dioxide in a sandstone-shale system[J]. Chemical Geology,2005,217(3/4):295-318.
[21] 刘娜. 砂岩对CO2的矿物捕获能力:来自松辽盆地南部红岗地区含片钠铝石砂岩的约束[D]. 长春:吉林大学,2011.
LIU N. Mineral trapping capacity estimation of CO2 in sandstones:constraints from the dawsonite-bearing sandstone in Honggang,southern part of Songliao Basin[D]. Changchun:Jilin University,2011.
[22] 周冰. 火山碎屑岩的CO2 矿物圈闭潜力研究:天然类似物与实验室实验约束[D].长春:吉林大学,2015.
ZHOU B. The potential capacity of CO2 mineral trapping in pyroclastic rock:constraints from natural analogue and experiments[D]. Changchun:Jilin University,2015.
[23] QU X L,LIU N L. Geology record of mantle-derived magmatogenetic CO2 gas in the northeastern China[J]. Acta Petrolei Sinica,2010,31(1):61-67.
[24] QU X Y,CHEN X,YU M,et al. Mineral dating of mantle-derived CO2 charging and its application in the southern Songliao Basin,China[J]. Applied Geochemistry,2016,68:19-28. doi: 10.1016/j.apgeochem.2016.03.005
[25] AHMAD A,WHEAT T A,CANADAY J D,et al. Processing and characterization of Na and (Na-K) beta-beta “alumina ceramics”[J]. Solid State Ionics,1994,68(3/4):233-241. doi: 10.1016/0167-2738(94)90181-3
[26] 范蕾蕾,叶俊伟,李鑫,等. NaAl(OH)2CO3阻燃晶须的水热合成及其阻燃性能[J]. 功能材料,2009,40(9):1580-1583.
FAN L L,YE J W,LI X,et al. Hydrothermal synthesis and flame-retardant properties of NaAl(OH)2CO3 whiskers[J]. Journal of Functional Materials,2009,40(9):1580-1583.
[27] STOICA G,ABELLÓ S O N,PÉREZ-RAMÍREZ J. Na-dawsonite derived aluminates for DMC production by transesterification of ethylene carbonate[J]. Applied Catalysis A:General,2009,365(2):252-260. doi: 10.1016/j.apcata.2009.06.022
[28] JUN C,SONG Y W,SHAN D Y,et al. Properties of dawsonite conversion film on AZ31 magnesium alloy[J]. Transactions of Nonferrous Metals Society of China,2011,21(4):936-942. doi: 10.1016/S1003-6326(11)60804-2
[29] LI X B,LIU N,ZHOU Q S,et al. Dawsonite preparation by deep carbonation decomposition of spent liquor from carbonation of sodium aluminate solutions[J]. Journal of Central South University (Science and Technology),2016,47(1):20-25.
[30] HERNANDEZ M J,ULIBARRI M A,CORNEJO J,et al. Thermal stability of aluminium hydroxycarbonates with monovalent cations[J]. Thermochimica Acta,1985,94(2):257-266. doi: 10.1016/0040-6031(85)85269-2
[31] KEENAN F J,HOWATSON J,SMITH J W. Thermal behavior of dawsonite[R]. Laramie:Laramie Energy Technology Center,1980.
[32] STOICA G,PÉREZ-RAMÍREZ J. Stability and inter-conversion of synthetic dawsonites in aqueous media[J]. Geochimica et Cosmochimica Acta,2010,74(24):7048-7058. doi: 10.1016/j.gca.2010.09.013
[33] PITSCH I,GEßNER W,BRÜCKNER A,et al. Synthesis and characterization of Fe2O3 containing aluminas by thermal decomposition of modified ammonium dawsonite[J]. Journal of Materials Chemistry,2001,11(10):2498-2503. doi: 10.1039/b101466h
[34] WU H T,SONG B,SUN Y,et al. Data mining technology in novel method for synthesis of sodium aluminium carbonate hydroxide[J]. CIESC Journal,2006,57(5):1236-1241.
[35] 姜求宇,吴文伟,廖森,等. 室温固相合成纳米碱式碳酸钠铝[J]. 应用化工,2005,34(2):99-101.
JIANG Q Y,WU W W,LIAO S,et al. Preparation of nano basic sodium aluminum carbonate by room temperature solid state reaction[J]. Applied Chemical Industry,2005,34(2):99-101.
[36] BÉNÉZETH P,PALMER D A,ANOVITZ L M,et al. Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements:implications for mineral trapping of CO2[J]. Geochimica et Cosmochimica Acta,2007,71(18):4438-4455. doi: 10.1016/j.gca.2007.07.003
[37] YANG Q H,LI D D,ZHUANG F C,et al. Transformation mechanism in preparation of pseudo-boehmite by NaAlO2 -CO2 method[J]. Chinese Journal of Catalysis,1997,18(6):478-482.
[38] 曲希玉,李倩,闫振,等. 固碳矿物—片钠铝石的最佳水热合成条件[J]. 中国石油大学学报(自然科学版),2023,47(3):27-34.
QU X Y,LI Q,YAN Z,et al. Optimum hydrothermal synthesis conditions of carbon fixing mineral-dawsonite[J]. Journal of China University of Petroleum (Edition of Natural Science),2023,47(3):27-34.
[39] 范泓澈,黄志龙,袁剑,等. 高温高压条件下甲烷和二氧化碳溶解度试验[J]. 中国石油大学学报(自然科学版),2011,35(2):6-11,19.
FAN H C,HUANG Z L,YUAN J,et al. Experiment on solubility of CH4 and CO2 at high temperature and high pressure[J]. Journal of China University of Petroleum (Edition of Natural Science),2011,35(2):6-11,19.
-



 下载:
下载: