Research progress and prospects of metal-dependent anaerobic methane oxidation in marine sediments
-
摘要:
海洋沉积物中大部分甲烷会通过甲烷厌氧氧化作用(anaerobic oxidation of methane, AOM)而被消耗。早期研究表明,AOM可与硫酸盐、硝酸盐和亚硝酸盐的还原作用相耦合,从而有效减少甲烷向大气的排放。最近,金属依赖型AOM(metal-AOM,活性金属氧化物还原反应驱动的AOM)被证实存在于自然界沉积物和富集培养的样品中。但是,目前仍未从自然海洋环境中分离获得能够介导metal-AOM的微生物。对海洋沉积物中metal-AOM的研究大多聚焦于热液或冷泉等海洋特殊生境,一系列研究表明地质流体在这些海底化能自养生态系统的维持和演化方面起到了重要作用,并深刻影响全球地球化学循环,因此,该科学问题研究吸引了越来越多的注意力。本文讨论了可能参与海洋沉积物中metal-AOM的微生物类群及其地球化学证据,并在前人工作基础上,以冲绳海槽冷泉-热液共生区为例,提出一种新的metal-AOM作用机制。认为在全球冷泉-热液系统相互作用地区的调查有助于更好地探讨metal-AOM的发生机制及微生物在深海生境中分布的连通性问题。
-
关键词:
- 海洋沉积物 /
- 金属依赖型甲烷厌氧氧化 /
- 冷泉-热液共生区 /
- 冲绳海槽
Abstract:A large fraction of methane is consumed by anaerobic oxidation (AOM) in marine sediments. Previous researches suggested that AOM is coupled to the reduction of sulfate, nitrate and nitrite, which may effectively reduce methane emission into the atmosphere. Recently, metal-dependent AOM (metal-AOM, AOM driven by active metal oxides reduction reaction) was demonstrated to occur in both the sediments in nature and enriched cultures. But the elusive microorganisms mediating metal-AOM process have not yet been isolated from natural marine environments, and most researches on metal-AOM in marine sediments focus on special marine habitats such as hydrothermal vents or cold seeps. However, a series of investigation shows that geological fluids play an important role in the maintenance and evolution of these submarine chemolithoautotrophy ecosystems, and profoundly affect the global geochemical cycle. Therefore, the research on this scientific problem has attracted more and more attention from marine scientists. In this review, the potential microbial communities and geochemical evidence of metal-AOM in marine sediments are summarized. On the basis of literature researches, taking the cold seeps and hydrothermal vents coexisted region, the Okinawa Trough, as an example, a new metal-AOM mechanism is proposed. The investigation in the global cold seeps and hydrothermal vents system interaction areas could be beneficial to better discuss the mechanism of metal-AOM and the connectivity of microbial distribution in deep-sea habitats.
-

-
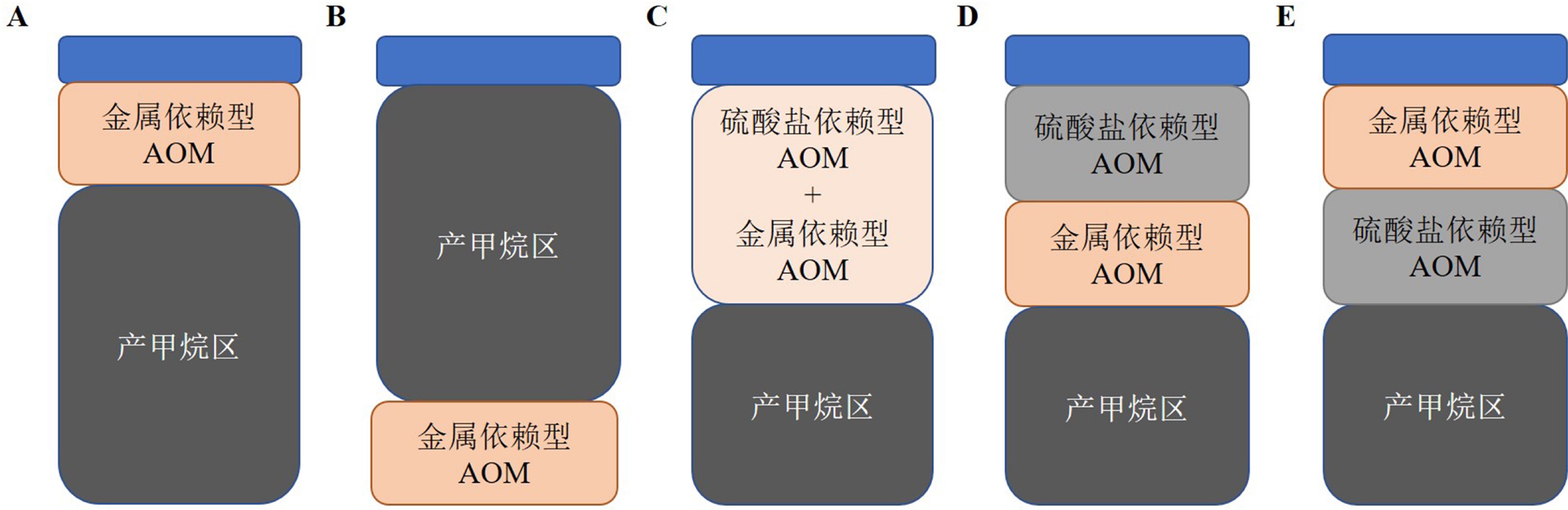
图 2 沉积物中metal-AOM潜在发生区模型[12]
Figure 2.
表 1 甲烷厌氧氧化作用类型及其标准吉布斯自由能(△G0’)
Table 1. Standard Gibbs free energies(△G0’)of different AOMs
类型 反应式 △G0’/(kJ/ molCH4) sulfate-AOM CH4+SO42− → HS−+HCO3−+H2O −16.3[7] NO3−-AOM CH4+4NO3− → HCO3−+H−+4NO2−+H2O −517.2[7] Fe-AOM CH4+8Fe(OH)3+16H+→ CO2+8Fe2++22H2O −571.2[7] Mn-AOM CH4+4MnO2+8H+ → CO2+4Mn2++6H2O −763.2[7] Cr-AOM CH4+4/3Cr2O72−+32/3H+ → 8/3Cr3++CO2+22/3H2O −841.4[7] NO2−-AOM CH4+8/3NO2−+8/3H+ → CO2+4/3N2+10/3H2O −928.0[8] 表 2 不同生态环境中metal-AOM潜在功能群
Table 2. Potential microbial communities of metal-AOM from different ecosystems
生态系统 采样地点 沉积带 数据来源 metal-AOM潜在功能群 海洋 Chowder Hill热液喷口 表层 富集培养 ANME-1c(Fe-AOM)[19] 圣塔莫尼卡海盆冷泉 表层 富集培养 ANME-2a、ANME-2c(Fe-AOM)[39] Eel River盆地冷泉 表层 富集培养 ANME-1、拟甲烷球菌属(Methanococcoides)/ANME-3(Mn-AOM)[35] Eel River 盆地冷泉 表层 富集培养 ANME-2(Mn-AOM)[36] Helgoland Mud 深层 富集培养 ANME-2a(Fe-AOM)[38] Helgoland Mud 表层 环境样品 JS1细菌、产甲烷古菌、甲烷盐菌属/ANME-3(Fe-AOM)[37] 淡水 Kinneret湖 深层 富集培养 甲烷八叠球菌目、甲基杆菌属(Fe-AOM)[26] 金溪水库(昆士兰州) 深层 富集培养 Candidatus Methanoperedens ferrireducens(Fe-AOM)[31] 金溪水库(布里斯班) 深层 富集培养 Candidatus Methanoperedens manganicus、Candidatus Methanoperedens manganireducens(Mn-AOM)[51] 受石油污染的含水层(伯米吉) 深层 环境样品 硫还原地杆菌(Geobacter sulfurreducens)(Fe-AOM)[53] Kabuno Bay 深层 环境样品 Methanoperedens(Fe-AOM)[52] Danish Lake Ørn 表层 富集培养 ANME-2d(Fe-AOM)[32] 陆地 泥火山(台湾东部) 表层 环境样品 ANME-2a、脱硫单胞菌属/居泥杆菌属(metal-AOM)[40] 室内 实验室培养 ANME-2d、奥奈达希瓦氏菌(metal-AOM)[54] -
[1] Moran M A. The global ocean microbiome [J]. Science, 2015, 350(6266): aac8455. doi: 10.1126/science.aac8455
[2] D’Hondt S, Pockalny R, Fulfer V M, et al. Subseafloor life and its biogeochemical impacts [J]. Nature Communication, 2019, 10(1): 3519. doi: 10.1038/s41467-019-11450-z
[3] Parkes R J, Cragg B A, Bale S J, et al. Deep bacterial biosphere in Pacific Ocean sediments [J]. Nature, 1994, 371(6496): 410-413. doi: 10.1038/371410a0
[4] 孙治雷, 魏合龙, 王利波, 等. 海底冷泉系统的碳循环问题及探测[J]. 应用海洋学学报, 2016, 35(3):442-450 doi: 10.3969/J.ISSN.2095-4972.2016.03.017
SUN Zhilei, WEI Helong, WANG Libo, et al. Focus issues of carbon cycle and detecting technologies in seafloor cold seepages [J]. Journal of Applied Oceanography, 2016, 35(3): 442-450. doi: 10.3969/J.ISSN.2095-4972.2016.03.017
[5] Hutchins D A, Fu F X. Microorganisms and ocean global change [J]. Nature Microbiology, 2017, 2: 17058. doi: 10.1038/nmicrobiol.2017.58
[6] Iversen N, Jørgensen B B. Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark) [J]. Limnology and Oceanography, 1985, 30(5): 944-955. doi: 10.4319/lo.1985.30.5.0944
[7] Timmers PHA, Welte CU, Koehorst JJ, et al. Reverse methanogenesis and respiration in methanotrophic archaea [J]. Archaea, 2017, 2017: 1654237.
[8] Norði K Á, Thamdrup B. Nitrate-dependent anaerobic methane oxidation in a freshwater sediment [J]. Geochimica et Cosmochimica Acta, 2014, 132: 141-150. doi: 10.1016/j.gca.2014.01.032
[9] Shen L D, Hu B L, Liu S, et al. Anaerobic methane oxidation coupled to nitrite reduction can be a potential methane sink in coastal environments [J]. Applied Microbiology and Biotechnology, 2016, 100(16): 7171-7180. doi: 10.1007/s00253-016-7627-0
[10] Zehnder A J B, Brock T D. Anaerobic methane oxidation: occurrence and ecology [J]. Applied and Environmental Microbiology, 1980, 39(1): 194-204. doi: 10.1128/AEM.39.1.194-204.1980
[11] Bau M, Dulski P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa [J]. Precambrian Research, 1996, 79(1-2): 37-55. doi: 10.1016/0301-9268(95)00087-9
[12] He Z F, Zhang Q Y, Feng Y D, et al. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane [J]. Science of the Total Environment, 2018, 610-611: 759-768. doi: 10.1016/j.scitotenv.2017.08.140
[13] Kvenvolden K A, Rogers B W. Gaia’s breath—global methane exhalations [J]. Marine and Petroleum Geology, 2005, 22(4): 579-590. doi: 10.1016/j.marpetgeo.2004.08.004
[14] Hoehler T M, Alperin M J, Albert D B, et al. Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium [J]. Global Biogeochemical Cycles, 1994, 8(4): 451-463. doi: 10.1029/94GB01800
[15] Hinrichs K U, Hayes J M, Sylva S P, et al. Methane-consuming archaebacteria in marine sediments [J]. Nature, 1999, 398(6730): 802-805. doi: 10.1038/19751
[16] Knittel K, Lösekann T, Boetius A, et al. Diversity and distribution of methanotrophic archaea at cold seeps [J]. Applied and Environmental Microbiology, 2005, 71(1): 467-479. doi: 10.1128/AEM.71.1.467-479.2005
[17] Brazelton W J, Schrenk M O, Kelley D S, et al. Methane- and sulfur-metabolizing microbial communities dominate the lost city hydrothermal field ecosystem [J]. Applied and Environmental Microbiology, 2006, 72(9): 6257-6270. doi: 10.1128/AEM.00574-06
[18] D'Hondt S, Rutherford S, Spivack A J. Metabolic activity of subsurface life in deep-sea sediments [J]. Science, 2002, 295(5562): 2067-2070. doi: 10.1126/science.1064878
[19] Bowles M W, Samarkin V A, Bowles K M, et al. Weak coupling between sulfate reduction and the anaerobic oxidation of methane in methane-rich seafloor sediments during ex situ incubation [J]. Geochimica et Cosmochimica Acta, 2011, 75(2): 500-519. doi: 10.1016/j.gca.2010.09.043
[20] Wankel S D, Adams M M, Johnston D T, et al. Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction [J]. Environmental Microbiology, 2012, 14(10): 2726-2740. doi: 10.1111/j.1462-2920.2012.02825.x
[21] Sivan O, Adler M, Pearson A, et al. Geochemical evidence for iron-mediated anaerobic oxidation of methane [J]. Limnology and Oceanography, 2011, 56(4): 1536-1544. doi: 10.4319/lo.2011.56.4.1536
[22] Ettwig K F, Butler M K, Le Paslier D, et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria [J]. Nature, 2010, 464(7288): 543-548. doi: 10.1038/nature08883
[23] Crowe S A, Katsev S, Leslie K, et al. The methane cycle in ferruginous Lake Matano [J]. Geobiology, 2011, 9(1): 61-78. doi: 10.1111/j.1472-4669.2010.00257.x
[24] Norði K Á, Thamdrup B, Schubert C J. Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment [J]. Limnology and Oceanography, 2013, 58(2): 546-554. doi: 10.4319/lo.2013.58.2.0546
[25] Riedinger N, Formolo M J, Lyons T W, et al. An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments [J]. Geobiology, 2014, 12(2): 172-181. doi: 10.1111/gbi.12077
[26] Raghoebarsing A A, Pol A, Van De Pas-Schoonen K, et al. A microbial consortium couples anaerobic methane oxidation to denitrification [J]. Nature, 2006, 440(7086): 918-921. doi: 10.1038/nature04617
[27] Bar-Or I, Elvert M, Eckert W, et al. Iron-coupled anaerobic oxidation of methane performed by a mixed bacterial-archaeal community based on poorly reactive minerals [J]. Environmental Science & Technology, 2017, 51(21): 12293-12301.
[28] Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process [J]. Annual Review of Microbiology, 2009, 63: 311-334. doi: 10.1146/annurev.micro.61.080706.093130
[29] Haroon M F, Hu S H, Shi Y, et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage [J]. Nature, 2013, 500(7464): 567-570. doi: 10.1038/nature12375
[30] Hu S H, Zeng R J, Burow L C, et al. Enrichment of denitrifying anaerobic methane oxidizing microorganisms [J]. Environmental Microbiology Reports, 2009, 1(5): 377-384. doi: 10.1111/j.1758-2229.2009.00083.x
[31] Ettwig K F, Zhu B L, Speth D, et al. Archaea catalyze iron-dependent anaerobic oxidation of methane [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(45): 12792-12796. doi: 10.1073/pnas.1609534113
[32] Cai C, Leu A O, Xie G J, et al. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe (III) reduction [J]. The ISME Journal, 2018, 12(8): 1929-1939. doi: 10.1038/s41396-018-0109-x
[33] Weber H S, Habicht K S, Thamdrup B. Anaerobic methanotrophic archaea of the ANME-2d cluster are active in a low-sulfate, iron-rich freshwater sediment [J]. Frontiers in Microbiology, 2017, 8: 619.
[34] Niu M Y, Fan X B, Zhuang G C, et al. Methane-metabolizing microbial communities in sediments of the Haima cold seep area, northwest slope of the South China Sea [J]. FEMS Microbiology Ecology, 2017, 93(9): fix101.
[35] Beal E J, House C H, Orphan V J. Manganese- and iron-dependent marine methane oxidation [J]. Science, 2009, 325(5937): 184-187. doi: 10.1126/science.1169984
[36] House C H, Beal E J, Orphan V J. The apparent involvement of ANMEs in mineral dependent methane oxidation, as an analog for possible martian methanotrophy [J]. Life, 2011, 1(1): 19-33. doi: 10.3390/life1010019
[37] Oni O, Miyatake T, Kasten S, et al. Distinct microbial populations are tightly linked to the profile of dissolved iron in the methanic sediments of the Helgoland mud area, North Sea [J]. Frontiers in Microbiology, 2015, 6: 365.
[38] Aromokeye D A, Kulkarni A C, Elvert M, et al. Rates and microbial players of iron-driven anaerobic oxidation of methane in methanic marine sediments [J]. Frontiers in Microbiology, 2020, 10: 3041. doi: 10.3389/fmicb.2019.03041
[39] Scheller S, Yu H, Chadwick G L, et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction [J]. Science, 2016, 351(6274): 703-707. doi: 10.1126/science.aad7154
[40] Chang Y H, Cheng T W, Lai W J, et al. Microbial methane cycling in a terrestrial mud volcano in eastern Taiwan [J]. Environmental Microbiology, 2012, 14(4): 895-908. doi: 10.1111/j.1462-2920.2011.02658.x
[41] Kato S, Hirai M, Ohkuma M, et al. Microbial metabolisms in an abyssal ferromanganese crust from the Takuyo-Daigo seamount as revealed by metagenomics [J]. PLoS One, 2019, 14(11): e0224888. doi: 10.1371/journal.pone.0224888
[42] Lipp J S, Morono Y, Inagaki F, et al. Significant contribution of Archaea to extant biomass in marine subsurface sediments [J]. Nature, 2008, 454(7207): 991-994. doi: 10.1038/nature07174
[43] D’Hondt S, Jørgensen B B, Miller D J, et al. Distributions of microbial activities in deep subseafloor sediments [J]. Science, 2004, 306(5705): 2216-2221. doi: 10.1126/science.1101155
[44] Vargas M, Kashefi K, Blunt-Harris E, et al. Microbiological evidence for Fe (III) reduction on early Earth [J]. Nature, 1998, 395(6697): 65-67. doi: 10.1038/25720
[45] Peng X T, Guo Z X, Chen S, et al. Formation of carbonate pipes in the northern Okinawa Trough linked to strong sulfate exhaustion and iron supply [J]. Geochimica et Cosmochimica Acta, 2017, 205: 1-13. doi: 10.1016/j.gca.2017.02.010
[46] Sun Z L, Wei H L, Zhang X H, et al. A unique Fe-rich carbonate chimney associated with cold seeps in the Northern Okinawa Trough, East China Sea [J]. Deep Sea Research Part I: Oceanographic Research Papers, 2015, 95: 37-53. doi: 10.1016/j.dsr.2014.10.005
[47] Xie R, Wu D D, Liu J, et al. Geochemical evidence of metal-driven anaerobic oxidation of methane in the Shenhu area, the South China Sea [J]. International Journal of Environmental Research and Public Health, 2019, 16(19): 3559. doi: 10.3390/ijerph16193559
[48] Liang L W, Wang Y Z, Sivan O, et al. Metal-dependent anaerobic methane oxidation in marine sediment: insights from marine settings and other systems [J]. Science China Life Sciences, 2019, 62(10): 1287-1295. doi: 10.1007/s11427-018-9554-5
[49] Canfield D E. Reactive iron in marine sediments [J]. Geochimica et Cosmochimica Acta, 1989, 53(3): 619-632. doi: 10.1016/0016-7037(89)90005-7
[50] Canfield D E, Raiswell R, Bottrell S H. The reactivity of sedimentary iron minerals toward sulfide [J]. American Journal of Science, 1992, 292(9): 659-683. doi: 10.2475/ajs.292.9.659
[51] Vigderovich H, Liang L W, Herut B, et al. Evidence for microbial iron reduction in the methanogenic sediments of the oligotrophic SE Mediterranean continental shelf [J]. Biogeosciences Discuss, 2019, 16: 1-25. doi: 10.5194/bg-16-1-2019
[52] Leu A O, Cai C, McIlroy S J, et al. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae [J]. The ISME Journal, 2020, 14(4): 1030-1041. doi: 10.1038/s41396-020-0590-x
[53] Amos R T, Bekins B A, Cozzarelli I M, et al. Evidence for iron-mediated anaerobic methane oxidation in a crude oil-contaminated aquifer [J]. Geobiology, 2012, 10(6): 506-517. doi: 10.1111/j.1472-4669.2012.00341.x
[54] Fu L, Li S W, Ding Z W, et al. Iron reduction in the DAMO/Shewanella oneidensis MR-1 coculture system and the fate of Fe (II) [J]. Water Research, 2016, 88: 808-815. doi: 10.1016/j.watres.2015.11.011
[55] McGlynn S E, Chadwick G L, Kempes C P, et al. Single cell activity reveals direct electron transfer in methanotrophic consortia [J]. Nature, 2015, 526(7574): 531-535. doi: 10.1038/nature15512
[56] Kletzin A, Heimerl T, Flechsler J, et al. Cytochromes c in archaea: distribution, maturation, cell architecture, and the special case of Ignicoccus hospitalis [J]. Frontiers in Microbiology, 2015, 6: 439.
[57] Roland F A E, Borges A V, Darchambeau F, et al. The possible occurrence of iron-dependent anaerobic methane oxidation in an Archean Ocean analogue [J]. Scientific Reports, 2021, 11(1): 1597. doi: 10.1038/s41598-021-81210-x
[58] Sivan O, Antler G, Turchyn A V, et al. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(40): E4139-E4147. doi: 10.1073/pnas.1412269111
[59] Egger M, Hagens M, Sapart C J, et al. Iron oxide reduction in methane-rich deep Baltic Sea sediments [J]. Geochimica et Cosmochimica Acta, 2017, 207: 256-276. doi: 10.1016/j.gca.2017.03.019
[60] Egger M, Rasigraf O, Sapart C J, et al. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments [J]. Environmental Science & Technology, 2015, 49(1): 277-283.
[61] Luo M, Torres M E, Hong W L, et al. Impact of iron release by volcanic ash alteration on carbon cycling in sediments of the northern Hikurangi margin [J]. Earth and Planetary Science Letters, 2020, 541: 116288. doi: 10.1016/j.jpgl.2020.116288
[62] Tagliabue A, Bopp L, Dutay J C, et al. Hydrothermal contribution to the oceanic dissolved iron inventory [J]. Nature Geoscience, 2010, 3(4): 252-256. doi: 10.1038/ngeo818
[63] McCollom T M, Shock E L. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems [J]. Geochimica et Cosmochimica Acta, 1997, 61(20): 4375-4391. doi: 10.1016/S0016-7037(97)00241-X
[64] Sun Z L, Wu N Y, Cao H, et al. Hydrothermal metal supplies enhance the benthic methane filter in oceans: An example from the Okinawa Trough [J]. Chemical Geology, 2019, 525: 190-209. doi: 10.1016/j.chemgeo.2019.07.025
[65] 吴能友, 孙治雷, 卢建国, 等. 冲绳海槽海底冷泉-热液系统相互作用[J]. 海洋地质与第四纪地质, 2019, 39(5):23-35
WU Nengyou, SUN Zhilei, LU Jianguo, et al. Interaction between seafloor cold seeps and adjacent hydrothermal activities in the Okinawa Trough [J]. Marine Geology & Quaternary Geology, 2019, 39(5): 23-35.
[66] McCollom T M. Geochemical constraints on primary productivity in submarine hydrothermal vent plumes [J]. Deep Sea Research Part I: Oceanographic Research Papers, 2000, 47(1): 85-101. doi: 10.1016/S0967-0637(99)00048-5
[67] Ruff S E, Bidle J F, Teske A P, et al. Global dispersion and local diversification of the methane seep microbiome [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(13): 4015-4020. doi: 10.1073/pnas.1421865112
[68] Hubert C, Loy A, Nickel M, et al. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed [J]. Science, 2009, 325(5947): 1541-1544. doi: 10.1126/science.1174012
[69] Schauer R, Bienhold C, Ramette A, et al. Bacterial diversity and biogeography in deep-sea surface sediments of the South Atlantic Ocean [J]. The ISME Journal, 2010, 4(2): 159-170. doi: 10.1038/ismej.2009.106
-




 下载:
下载: