The impact of different chemical leaching methods on REE and Sr-Nd isotopes analysis of sediment detrital components
-
摘要:
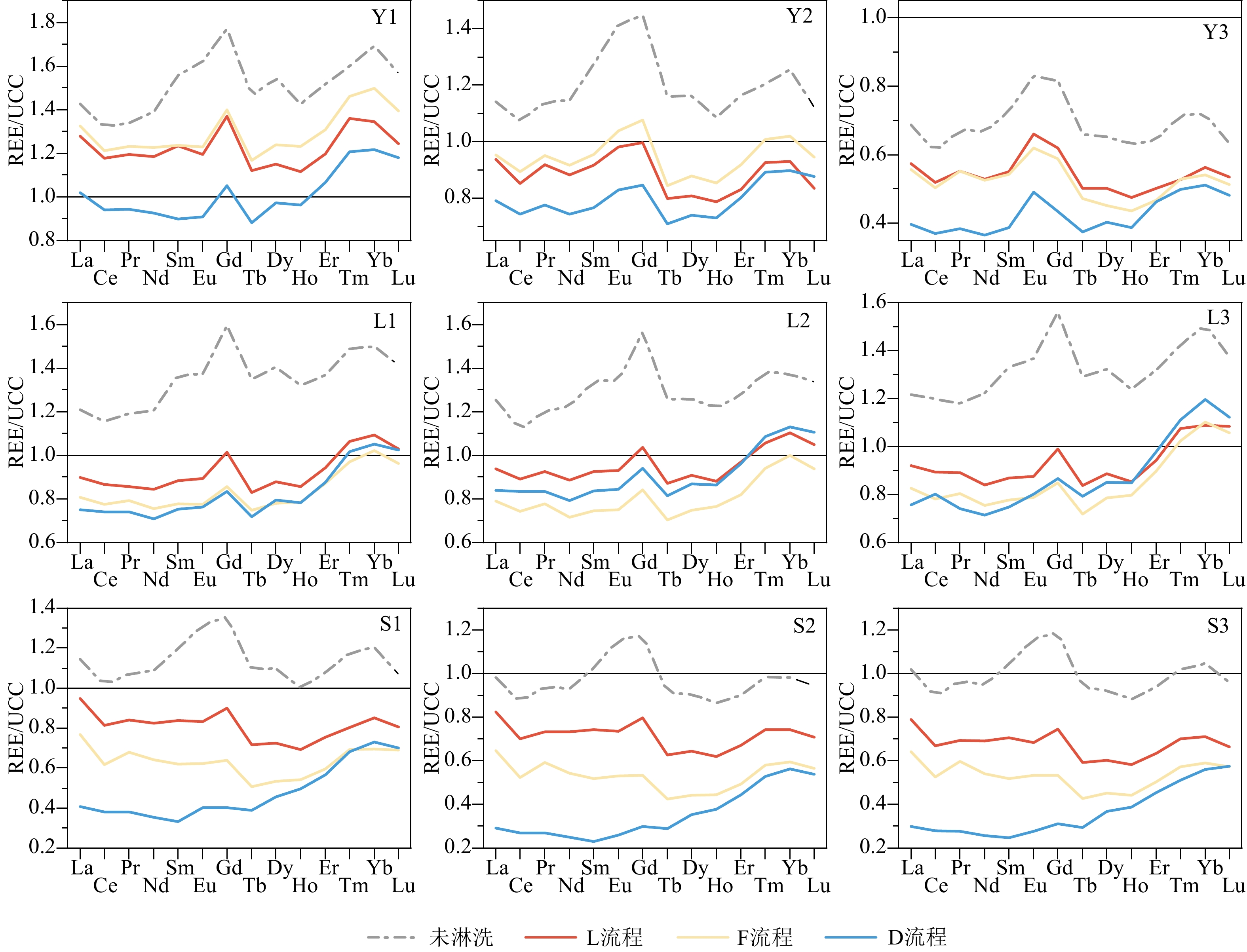
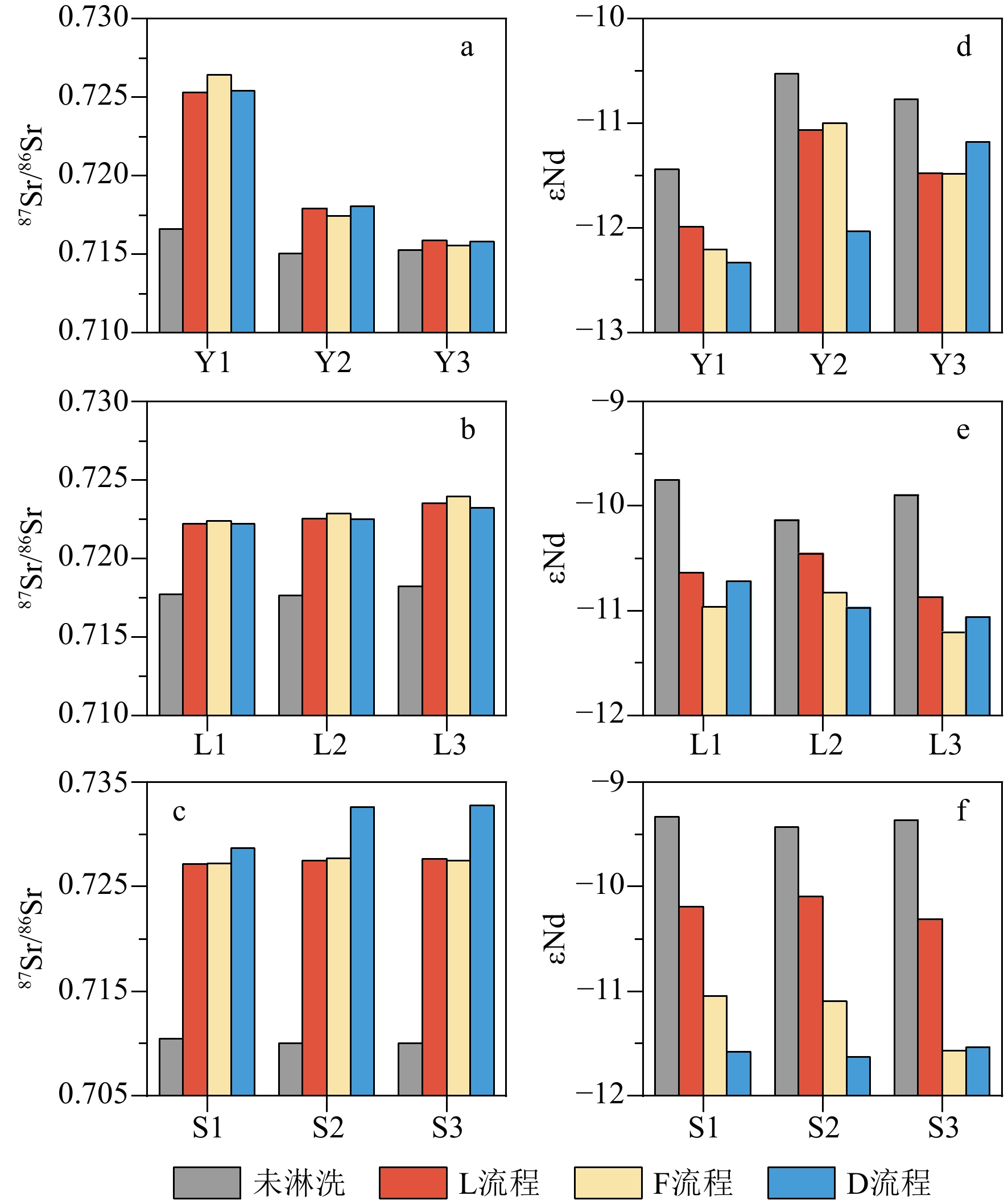
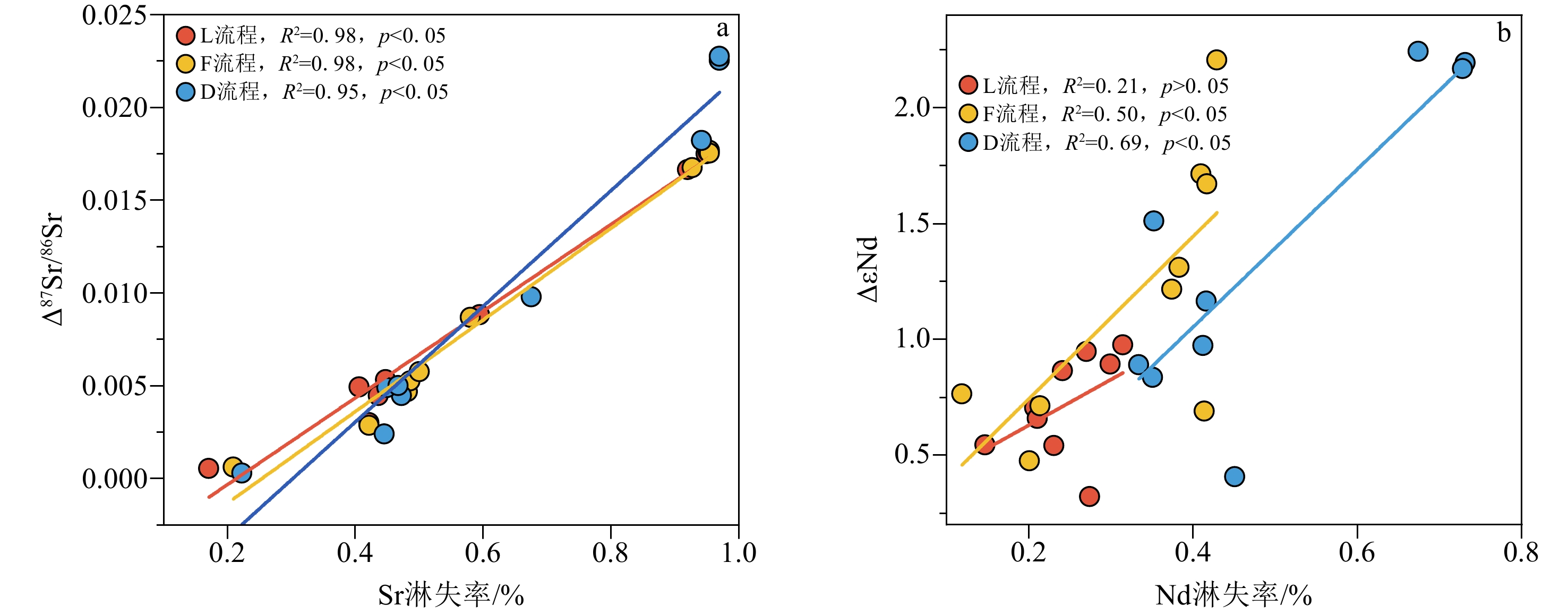
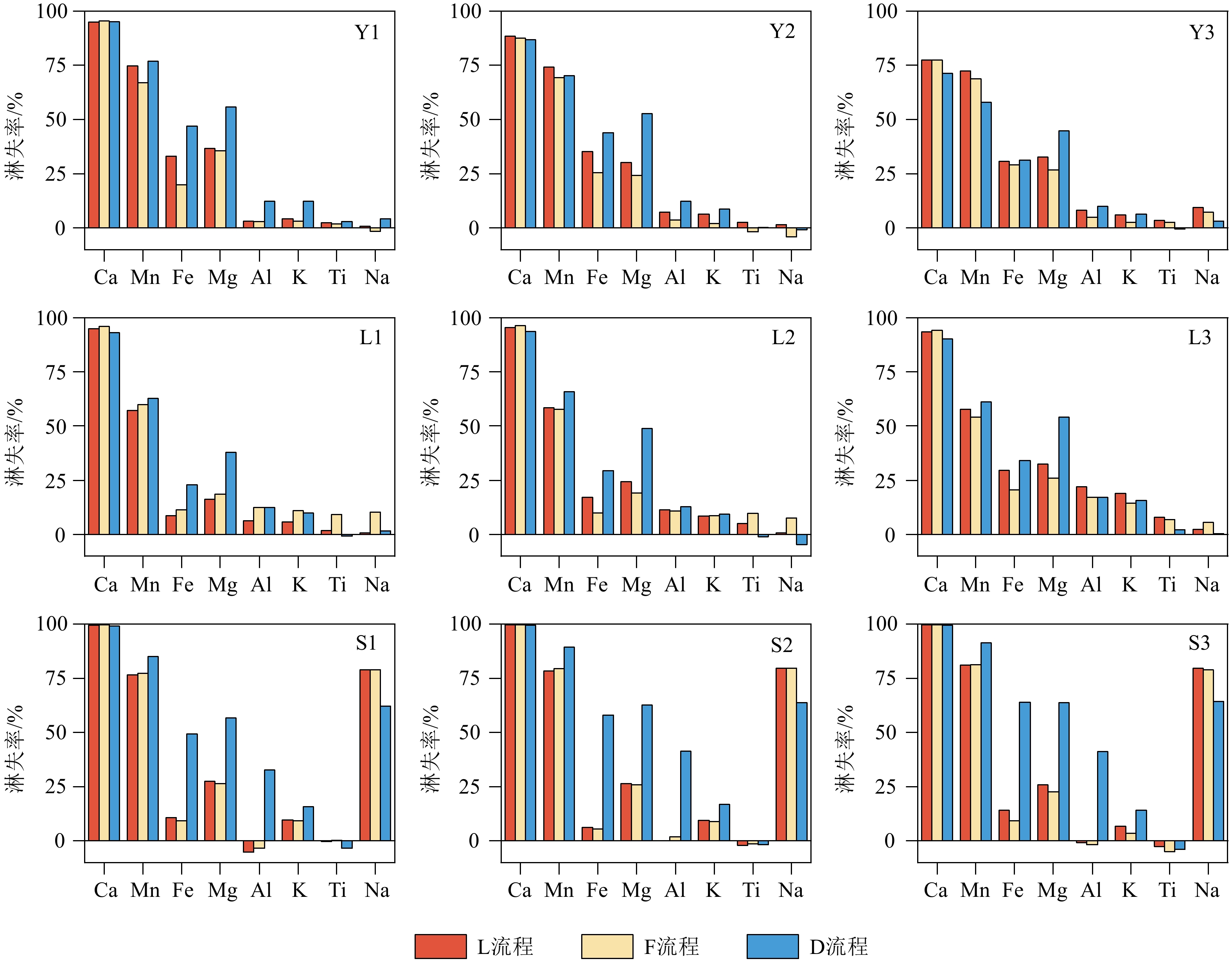
沉积物是一种成分复杂的混合物,不同地学研究往往需要提取沉积物中不同的化学组分。顺序淋滤是区分沉积物不同组分较为常用的方法,但目前开展的顺序淋滤实验往往只针对单一类型沉积物样品开展探索,其淋滤效果是否也广泛适用其他类型沉积物样品并不清楚。本研究选取了来自长江、黄土高原以及南海的3种不同类型沉积物样品,借鉴目前应用较多的两种顺序淋滤流程以及本文改进的流程共计3种方法对样品进行淋洗,评估不同淋洗方法对样品中碎屑组分REE和Sr-Nd同位素测试的影响。研究证实1.5 mol/L盐酸会过度去除样品中的碳酸盐组分,导致部分主量元素(Mn、Fe和Mg)和REE元素的损失达50%以上,还可能会造成包括黏土矿物在内的部分硅酸盐碎屑组分溶解;酸性较适中的1 mol/L醋酸钠缓冲溶液更利于准确去除沉积物中的碳酸盐组分。非碎屑组分的去除会导致沉积物Sr同位素升高,影响碎屑组分Sr同位素组成的主要是碳酸盐组分。对Nd同位素,非碎屑组分的去除会导致沉积物碎屑组分ɛNd降低1—2个单位,但是Nd的淋失率与碎屑组分ɛNd的变化关系更加复杂,过度的震荡和延长反应时间对不同类型沉积物ɛNd的影响机制仍有待进一步研究。
Abstract:Sediments are complex mixtures, and different geological studies often require the extraction of different chemical components from sediments. Sequential leaching is a commonly used method to differentiate different components of sediments. However, the effectiveness of sequential leaching experiments conducted so far is often explored only for a single type of sediment sample. It is unclear whether the leaching effect is also widely applicable to other types of sediment samples. In this study, three types of sediment samples from the Yangtze River, the Loess Plateau, and the South China Sea were selected. Three methods, including two commonly used sequential leaching procedures and an improved procedure proposed in this study, were applied to leach the samples. The aim was to assess the impact of different leaching methods on the REE and Sr-Nd isotopes analysis of the sediment detrital components. Results show that 1.5 M hydrochloric acid tends to excessively remove carbonate components from the samples, leading to a loss of over 50% of some major elements (Mn, Fe, and Mg) and REE elements. It could also cause the dissolution of some silicate detrital components, including clay minerals. 1 M sodium acetate buffer solution with moderate acidity was shown more favorable for accurately removing carbonate components from all sediments. The removal of non-detrital components resulted in an increase of Sr isotopes in all the sediments. The main component influencing the isotopic composition of Sr in detrital components was the carbonate fraction. For Nd isotopes, the removal of non-detrital components led to a decrease in ɛNd of sediment detrital components by 1—2 units. However, the relationship between the loss of Nd and the changes in detrital component ɛNd is more complex, and the effects of excessive shaking and prolonged reaction time on the ɛNd of different types of sediments still require further investigation.
-
Key words:
- chemical leaching /
- non-detrital components /
- REE /
- Sr-Nd isotopes /
- comparative methods
-

-
表 1 样品信息
Table 1. Samples information
编号 样品 位置 岩性 Y1 31°48′16″N、121°19′35″E 黄色粉砂质黏土,重矿物以辉石和磁铁矿为特征 Y2 长江沉积物 灰黑色粉砂质砾石,重矿物以角闪石和石榴石为特征 Y3 灰黄色砂质砾石,重矿物以角闪石和石榴石为特征 L1 35°00′33″N、107°30′33″E 褐色粉砂,矿物以石英、长石为主 L2 黄土沉积物 L3 红色粉砂,矿物以石英、长石为主 S1 9°21′43″N、113°17′7″E S2 南海沉积物 黏土,矿物以黏土矿物和碳酸盐为主 S3 表 2 沉积物碎屑组分提取方法
Table 2. Leaching methods for sediment detrital components
Dosseto等[8]
(简称为D流程)Francke等[11]
(简称为F流程)本文流程
(简称L流程)可交换态 ①8 mL 1 M的 Mg(NO3)2,室温条件下
超声3 min①8 mL 1 M 的Mg(NO3)2,室温条件下振荡箱摇晃50 min,手动摇晃30 min 可酸溶态 ②20 mL 1.5 M HCl ,室温条件下手动
摇晃6 h②20 mL 1M的 CH3COONa(pH = 5,CH3COOH调节),室温条件下超声
反应5 min×3②10 mL 1 M 的CH3COONa(pH = 5,CH3COOH调节),室温条件下振荡箱摇
晃5 h,期间每隔30 min手动摇晃一次可还原态 ③20 mL 0.04 M NH2OH·HCl (pH = 2, CH3COOH调节),室温条件下手动摇晃 6 h ③20 mL 0.1 M 的NH2OH·HCl (pH = 2, CH3COOH调节),80 ℃条件下超声
反应6 min × 2③20 mL 0.1 M 的NH2OH·HCl (pH = 2, CH3COOH调节),90℃条件下保温反应3 h,期间每隔30 min 摇晃一次并超声5 min 可氧化态 ①置入马弗炉550 ℃ 灼烧8 h ④20 mL 15% H2O2(pH = 2,0.02 M HNO3调节),80℃条件下超声反应
3 min × 2;
5 mL 3.2 M的 NH4OAc(通过20% HNO3溶解),室温条件下手动摇晃1 min④5 mL 30% 的H2O2,80℃条件下保温反应
3 h,期间每隔30 min 摇晃一次并超声5 min注:①表示淋洗流程中的第一步,后续序号以此类推。 表 3 样品质量淋失率
Table 3. The leaching mass loss rate of the samples
样品编号 质量损失率/% D流程 F流程 L流程 平均值 Y1 25.6 18.15 19.22 15.20 Y2 21.8 14.17 16.27 Y3 7.68 7.2 6.7 L1 25.35 26.37 21.69 23.73 L2 24.45 23.77 22.49 L3 20.44 24.08 24.97 S1 51.05 35.48 33.98 47.24 S2 62.37 46.11 43.53 S3 62.05 45.62 44.96 -
[1] Tessier A, Campbell P G C, Bisson M. Sequential extraction procedure for the speciation of particulater trace metalselements[J]. Analytical Chemistry, 1979, 51(7):844-851. doi: 10.1021/ac50043a017
[2] Rauret G, Rubio R, López-Sánchez J F. Optimization of Tessier procedure for metal solid speciation in river sediments[J]. International Journal of Environmental Analytical Chemistry, 1989, 36(2):69-83. doi: 10.1080/03067318908026859
[3] Rauret G, López-Sánchez J F, Sahuquillo A, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials[J]. Journal of Environmental Monitoring, 1999, 1(1):57-61. doi: 10.1039/a807854h
[4] Ure A M, Quevauviller P, Muntau H, et al. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities[J]. International Journal of Environmental Analytical Chemistry, 1993, 51(1-4):135-151. doi: 10.1080/03067319308027619
[5] Poulton S W, Canfield D E. Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates[J]. Chemical Geology, 2005, 214(3-4):209-221. doi: 10.1016/j.chemgeo.2004.09.003
[6] Bayon G, German C R, Boella R M, et al. An improved method for extracting marine sediment fractions and its application to Sr and Nd isotopic analysis[J]. Chemical Geology, 2002, 187(3-4):179-199. doi: 10.1016/S0009-2541(01)00416-8
[7] Schultz M K, Burnett W C, Inn K G W. Evaluation of a sequential extraction method for determining actinide fractionation in soils and sediments[J]. Journal of Environmental Radioactivity, 1998, 40(2):155-174. doi: 10.1016/S0265-931X(97)00075-1
[8] Dosseto A, Hesse P P, Maher K, et al. Climatic and vegetation control on sediment dynamics during the last glacial cycle[J]. Geology, 2010, 38(5):395-398. doi: 10.1130/G30708.1
[9] Depaolo D J, Maher K, Christensen J N, et al. Sediment transport time measured with U-series isotopes: results from ODP North Atlantic drift site 984[J]. Earth and Planetary Science Letters, 2006, 248(1-2):394-410. doi: 10.1016/j.jpgl.2006.06.004
[10] Li L, Liu X J, Li T, et al. Uranium comminution age tested by the eolian deposits on the Chinese Loess Plateau[J]. Earth and Planetary Science Letters, 2017, 467:64-71. doi: 10.1016/j.jpgl.2017.03.014
[11] Francke A, Carney S, Wilcox P, et al. Sample preparation for determination of comminution ages in lacustrine and marine sediments[J]. Chemical Geology, 2018, 479:123-135. doi: 10.1016/j.chemgeo.2018.01.003
[12] Chen J F, Li C, Galy A, et al. Uranium-series comminution ages constrain large catchment erosion and its response to climate change: a case study from the Changjiang[J]. Earth and Planetary Science Letters, 2024, 625:118493. doi: 10.1016/j.jpgl.2023.118493
[13] 骆正骅, 李超, 赖正, 等. 树脂柱串联法分离地质样品中Sr-Nd-U[J]. 岩矿测试, 2023, 42(1):102-113 doi: 10.3969/j.issn.0254-5357.2023.1.ykcs202301007
LUO Zhenghua, LI Chao, LAI Zheng, et al. Separation of Sr, Nd, and U from geological samples using tandem resin column[J]. Rock and Mineral Analysis, 2023, 42(1):102-113.] doi: 10.3969/j.issn.0254-5357.2023.1.ykcs202301007
[14] Rudnick R L, Gao S. 3.01 - Composition of the continental crust[M]// Holland H D, Turekian K K. Treatise on Geochemistry. Oxford: Pergamon, 2003, 3: 1-64.
[15] Haese R R, Wallmann K, Dahmke A, et al. Iron species determination to investigate early diagenetic reactivity in marine sediments[J]. Geochimica et Cosmochimica Acta, 1997, 61(1):63-72. doi: 10.1016/S0016-7037(96)00312-2
[16] Whiteley J D, Pearce N J G. Metal distribution during diagenesis in the contaminated sediments of Dulas Bay, Anglesey, N. Wales, UK[J]. Applied Geochemistry, 2003, 18(6):901-913. doi: 10.1016/S0883-2927(02)00183-X
[17] Lyons T W, Severmann S. A critical look at iron paleoredox proxies: new insights from modern euxinic marine basins[J]. Geochimica et Cosmochimica Acta, 2006, 70(23):5698-5722. doi: 10.1016/j.gca.2006.08.021
[18] Canfield D E. Reactive iron in marine sediments[J]. Geochimica et Cosmochimica Acta, 1989, 53(3):619-632. doi: 10.1016/0016-7037(89)90005-7
[19] Aller R C, Blair N E. Sulfur diagenesis and burial on the Amazon shelf: major control by physical sedimentation processes[J]. Geo-Marine Letters, 1996, 16(1):3-10. doi: 10.1007/BF01218830
[20] Raiswell R, Canfield D E. Sources of iron for pyrite Formation in marine sediments[J]. American Journal of Science, 1998, 298(3):219-245. doi: 10.2475/ajs.298.3.219
[21] Poulton S W, Raiswell R. The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition[J]. American Journal of Science, 2002, 302(9):774-805. doi: 10.2475/ajs.302.9.774
[22] Zhu M X, Hao X C, Shi X N, et al. Speciation and spatial distribution of solid-phase iron in surface sediments of the East China Sea continental shelf[J]. Applied Geochemistry, 2012, 27(4):892-905. doi: 10.1016/j.apgeochem.2012.01.004
[23] Balikungeri A, Robin D, Haerdi W. Manganese in natural waters I. Speciation of manganese in running waters and sediments[J]. International Journal of Environmental Analytical Chemistry, 1985, 19(3):227-241.
[24] Wan S M, Li A C, Clift P D, et al. Development of the East Asian summer monsoon: evidence from the sediment record in the South China Sea since 8.5 Ma[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2006, 241(1):139-159. doi: 10.1016/j.palaeo.2006.06.013
[25] Meng X Q, Liu L W, Wang X T, et al. Mineralogical evidence of reduced East Asian summer monsoon rainfall on the Chinese loess plateau during the early Pleistocene interglacials[J]. Earth and Planetary Science Letters, 2018, 486:61-69. doi: 10.1016/j.jpgl.2017.12.048
[26] 范德江, 杨作升, 王文正. 长江、黄河沉积物中碳酸盐组成及差异[J]. 自然科学进展, 2002, 12(1):60-6462-66 doi: 10.3321/j.issn:1002-008X.2002.01.013
FAN Dejiang, YANG Zuosheng, WANG Wenzheng. Carbonate composition and differences in sediments of Yangtze River and Yellow River[J]. Progress in Chinese Natural Sciences, 2002, 12(1):60-6462-66.] doi: 10.3321/j.issn:1002-008X.2002.01.013
[27] Yue W, Jin B F, Zhao B C. Transparent heavy minerals and magnetite geochemical composition of the Yangtze River sediments: implication for provenance evolution of the Yangtze Delta[J]. Sedimentary Geology, 2018, 364:42-52. doi: 10.1016/j.sedgeo.2017.12.006
[28] Yue W, Yang S Y, Zhao B C, et al. Changes in environment and provenance within the Changjiang (Yangtze River) Delta during Pliocene to Pleistocene transition[J]. Marine Geology, 2019, 416:105976. doi: 10.1016/j.margeo.2019.105976
[29] Chauhan O S, Gujar A R, Rao C M. On the occurrence of ferromanganese micronodules from the sediments of the Bengal Fan: a high terrigenous sediment input region[J]. Earth and Planetary Science Letters, 1994, 128(3-4):563-573. doi: 10.1016/0012-821X(94)90170-8
[30] Barling J, Arnold G L, Anbar A D. Natural mass-dependent variations in the isotopic composition of molybdenum[J]. Earth and Planetary Science Letters, 2001, 193(3-4):447-457. doi: 10.1016/S0012-821X(01)00514-3
[31] Bayon G, German C R, Burton K W, et al. Sedimentary Fe-Mn oxyhydroxides as paleoceanographic archives and the role of aeolian flux in regulating oceanic dissolved REE[J]. Earth and Planetary Science Letters, 2004, 224(3-4):477-492. doi: 10.1016/j.jpgl.2004.05.033
[32] Nesbitt H W. Mobility and fractionation of rare earth elements during weathering of a granodiorite[J]. Nature, 1979, 279(5710):206-210. doi: 10.1038/279206a0
[33] Schroeder J O, Murray R W, Leinen M, et al. Barium in equatorial Pacific carbonate sediment: terrigenous, oxide, and biogenic associations[J]. Paleoceanography, 1997, 12(1):125-146. doi: 10.1029/96PA02736
[34] Zhang C S, Wang L J, Zhang S, et al. Geochemistry of rare earth elements in the mainstream of the Yangtze River, China[J]. Applied Geochemistry, 1998, 13(4):451-462. doi: 10.1016/S0883-2927(97)00079-6
[35] 盛雪芬, 杨杰东, 李春雷, 等. 黄土和沉积岩中分离方解石和白云石的方法实验[J]. 岩矿测试, 2000, 19(4):264-267 doi: 10.3969/j.issn.0254-5357.2000.04.006
SHENG Xuefen, YANG Jiedong, LI Chunlei, et al. A method for separation of calcite and dolomite in loess and sedimentary rocks[J]. Rock and Mineral Analysis, 2000, 19(4):264-267.] doi: 10.3969/j.issn.0254-5357.2000.04.006
[36] 邹亮, 韦刚健. 顺序提取法探讨沉积物中主量元素在不同相态的分配特征[J]. 海洋地质与第四纪地质, 2007, 27(2):133-140
ZOU Liang, WEI Gangjian. Distribution of major elements in sediment by sequential extraction procedures[J]. Marine Geology & Quaternary Geology, 2007, 27(2):133-140.]
[37] McLennan S M. Chapter 7. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes[M]. //Lipin B R, McKay G A. Geochemistry and Mineralogy of Rare Earth Elements. Berlin, Boston: De Gruyter, 1989, 21(1):169-200.
[38] Alshameri A, He H P, Xin C, et al. Understanding the role of natural clay minerals as effective adsorbents and alternative source of rare earth elements: adsorption operative parameters[J]. Hydrometallurgy, 2019, 185:149-161. doi: 10.1016/j.hydromet.2019.02.016
[39] Borst A M, Smith M P, Finch A A, et al. Adsorption of rare earth elements in regolith-hosted clay deposits[J]. Nature Communications, 2020, 11(1):4386. doi: 10.1038/s41467-020-17801-5
[40] Tostevin R, Shields G A, Tarbuck G M, et al. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings[J]. Chemical Geology, 2016, 438:146-162. doi: 10.1016/j.chemgeo.2016.06.027
[41] Zhao Y Y, Wei W, Li S Z, et al. Rare earth element geochemistry of carbonates as a proxy for deep-time environmental reconstruction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2021, 574:110443. doi: 10.1016/j.palaeo.2021.110443
[42] Zhao Y Y, Wei W, Santosh M, et al. A review of retrieving pristine rare earth element signatures from carbonates[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2022, 586:110765. doi: 10.1016/j.palaeo.2021.110765
[43] Freslon N, Bayon G, Toucanne S, et al. Rare earth elements and neodymium isotopes in sedimentary organic matter[J]. Geochimica et Cosmochimica Acta, 2014, 140:177-198. doi: 10.1016/j.gca.2014.05.016
[44] 杨守业, 王中波. 长江主要支流与干流沉积物的REE组成[J]. 矿物岩石地球化学通报, 2011, 30(1):31-39 doi: 10.3969/j.issn.1007-2802.2011.01.005
YANG Shouye, WANG Zhongbo. Rare earth element compositions of the sediments from the major tributaries and the main stream of the Changjiang River[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2011, 30(1):31-39.] doi: 10.3969/j.issn.1007-2802.2011.01.005
[45] 周斌, 古再丽努尔·外力, Peterse France, 等. 黄土高原中部450ka以来植被演化的有机碳同位素与分子化石记录[J]. 中国科学: 地球科学, 2016, 46(4): 509-518
Zhou Bin, Guzalnur Wali G, Peterse F, et al. Organic carbon isotope and molecular fossil records of vegetation evolution in central Loess Plateau since 450 kyr[J]. Science China Earth Sciences, 2016, 59(6): 1206-121546(4): 509-518.]
[46] 杨守业, 李从先. 长江三角洲晚新生代沉积物有机碳、总氮和碳酸盐组成及古环境意义[J]. 地球化学, 2006, 35(3):249-256 doi: 10.3321/j.issn:0379-1726.2006.03.004
YANG Shouye, LI Congxian. Compositions of organic elements and carbonate in the Late Cenozoic sediments of the Changjiang Delta: implication for paleoenvironmental changes[J]. Geochimica, 2006, 35(3):249-256.] doi: 10.3321/j.issn:0379-1726.2006.03.004
[47] Dasch E J. Strontium isotopes in weathering profiles, deep-sea sediments, and sedimentary rocks[J]. Geochimica et Cosmochimica Acta, 1969, 33(12):1521-1552. doi: 10.1016/0016-7037(69)90153-7
[48] Goldstein S L, O'Nions R K, Hamilton P J. A Sm-Nd isotopic study of atmospheric dusts and particulates from major river systems[J]. Earth and Planetary Science Letters, 1984, 70(2):221-236. doi: 10.1016/0012-821X(84)90007-4
[49] Borg L E, Banner J L. Neodymium and strontium isotopic constraints on soil sources in Barbados, West Indies[J]. Geochimica et Cosmochimica Acta, 1996, 60(21):4193-4206. doi: 10.1016/S0016-7037(96)00252-9
[50] Goldstein S J, Jacobsen S B. Nd and Sr Isotopic Systematics of river water suspended material: - implications for crustal evolution[J]. Earth and Planetary Science Letters, 1988, 87(3):249-265. doi: 10.1016/0012-821X(88)90013-1
[51] Abbott A N, Löhr S C, Payne A, et al. Widespread lithogenic control of marine authigenic neodymium isotope records? Implications for paleoceanographic reconstructions[J]. Geochimica et Cosmochimica Acta, 2022, 319:318-336. doi: 10.1016/j.gca.2021.11.021
[52] 赖正. 长江口放射成因锶同位素的地球化学行为[D]. 上海: 同济大学, 2021
LAI Zheng. Geochemical behavior of radiogenic strontium isotopes in the Changjiang River estuary[D]. Shanghai: Tongji University, 2021.]
[53] Tripathy G R, Singh S K, Krishnaswami S. Sr and Nd isotopes as tracers of chemical and physical erosion[M]//Baskaran M. Handbook of Environmental Isotope Geochemistry. Berlin, Heidelberg: Springer Berlin Heidelberg, 20121: 521-552.
[54] Garçon M, Chauvel C, France-Lanord C, et al. Which minerals control the Nd–Hf–Sr–Pb isotopic compositions of river sediments?[J]. Chemical Geology, 2014, 364:42-55. doi: 10.1016/j.chemgeo.2013.11.018
-



 下载:
下载: