Separation of Iron(II) and Vanadium(IV)、Chromium(III) from Low Valence Vanadium Solution and Preparation of Mohr's Salt
-
摘要:
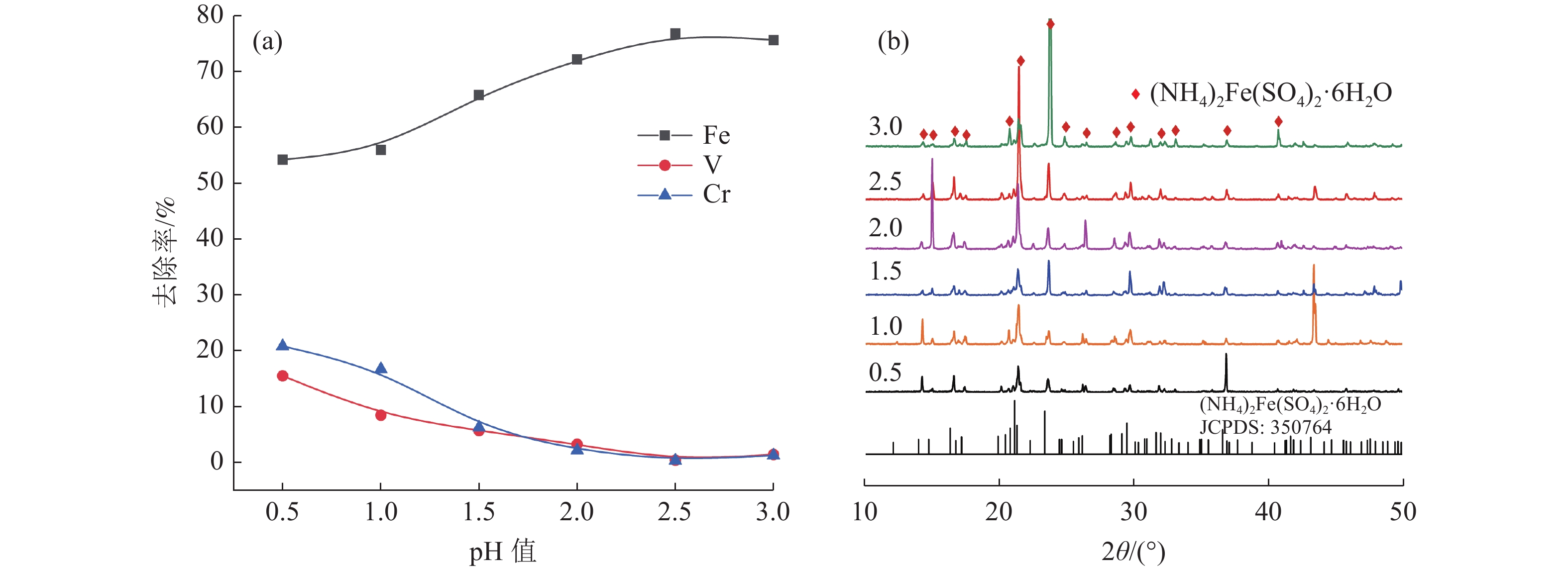
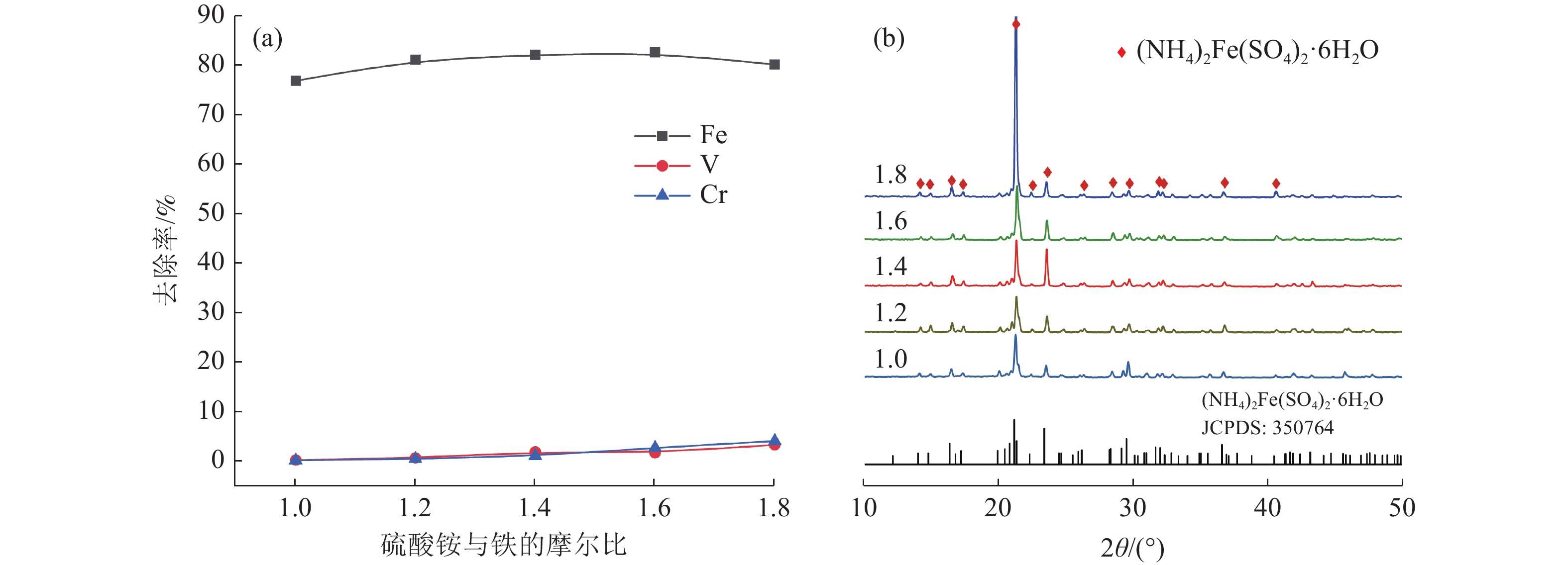
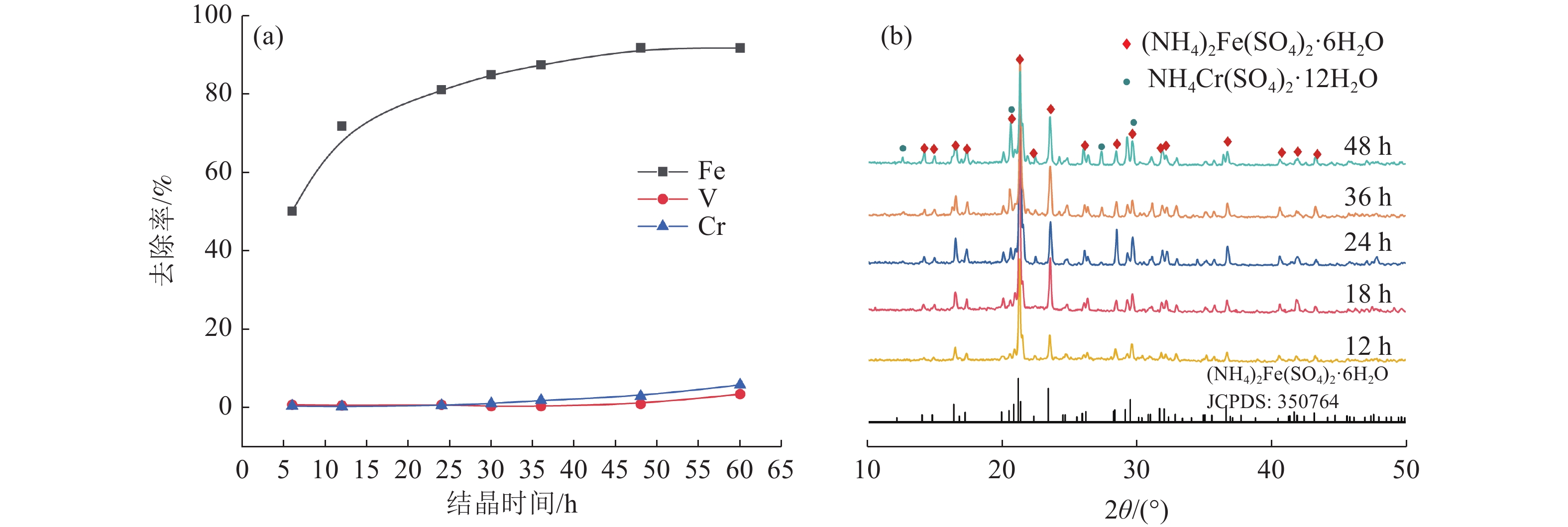
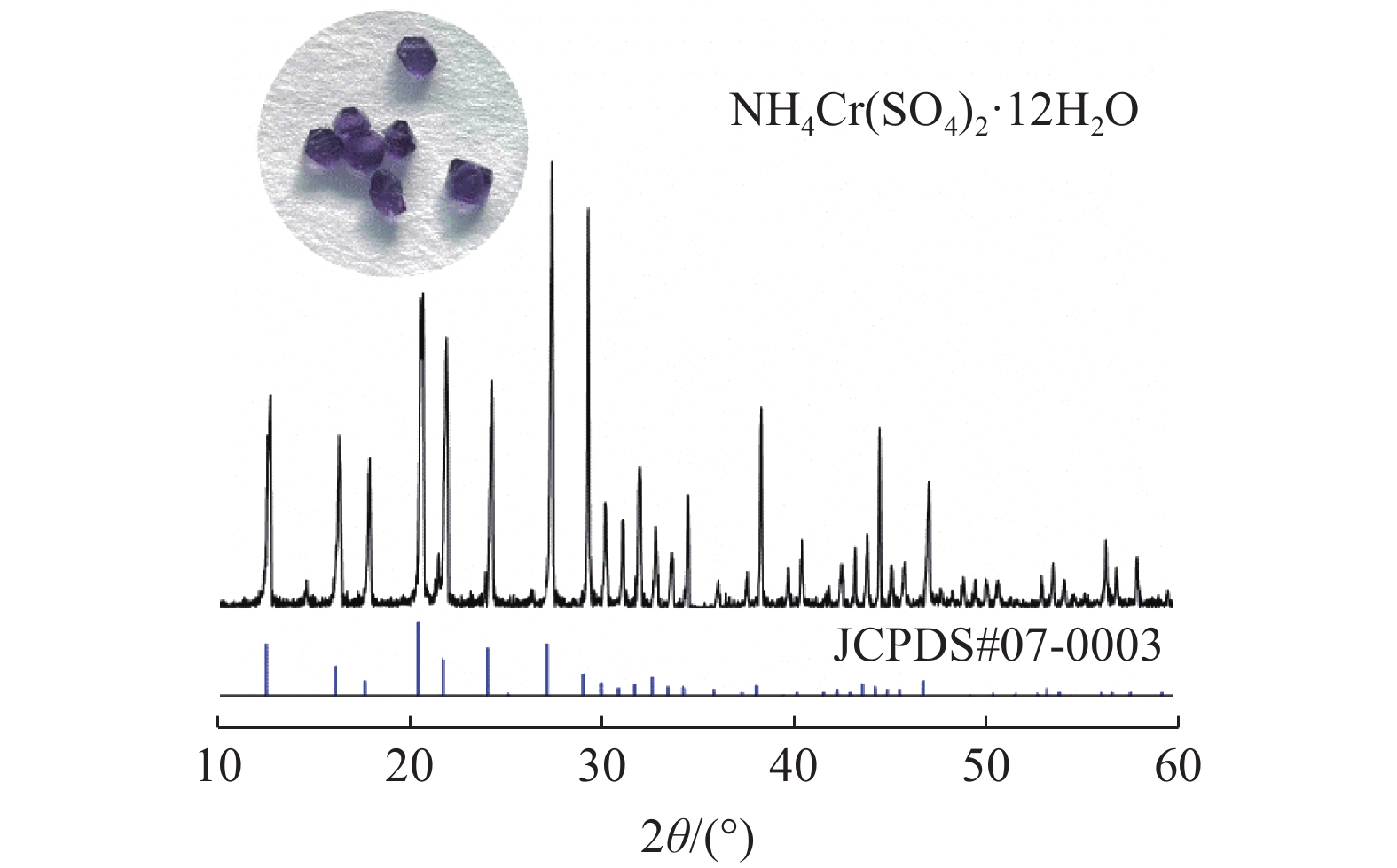
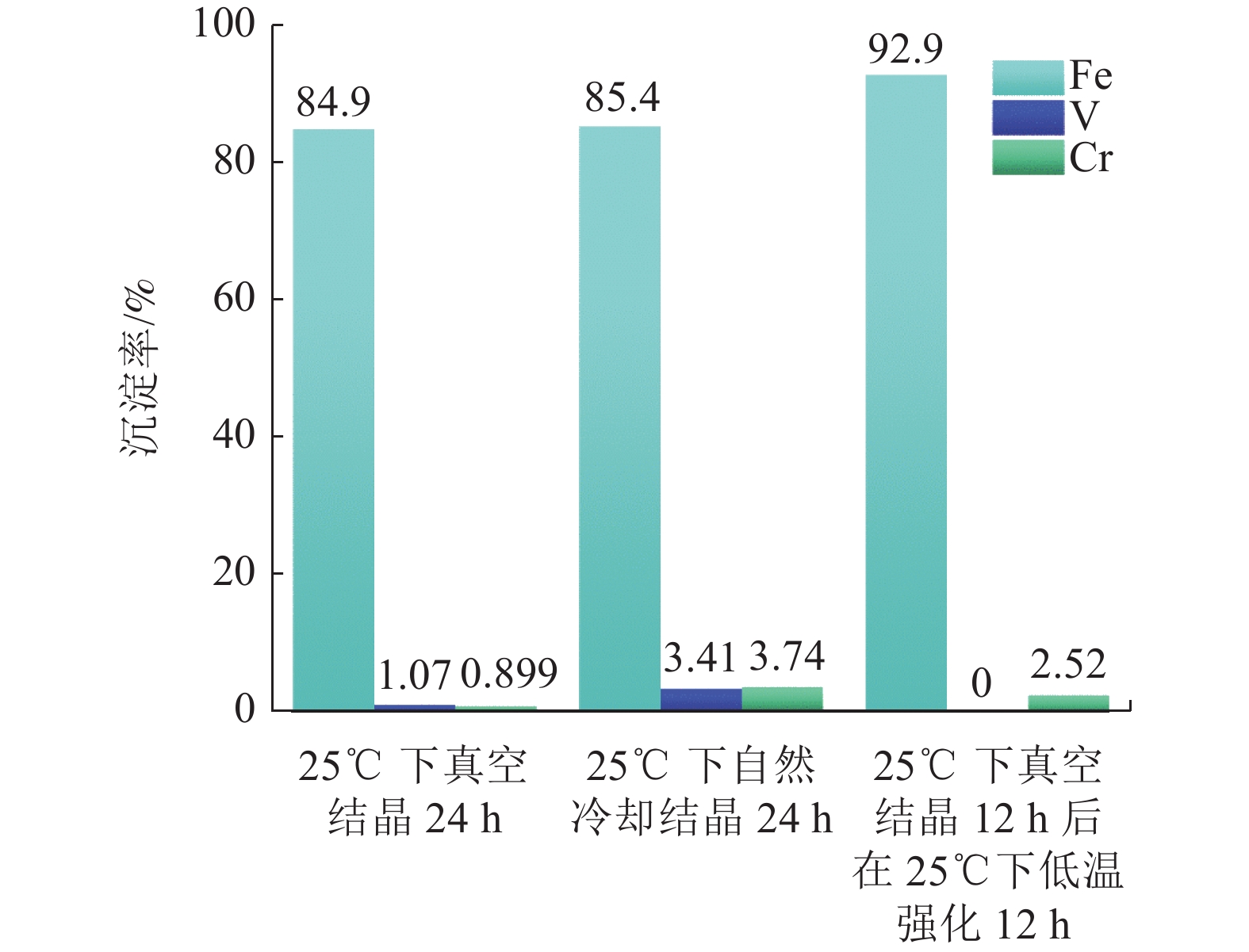
本文以钒渣提取低价钒过程中得到的含低价钒、铬和铁的浸出液为研究对象,将真空冷却法应用于钒铁分离,铁从浸出液中以莫尔盐结晶析出,实现了铁与低价态钒、铬的高效分离并制备了莫尔盐。探究了pH值、硫酸铵添加量、反应时间、结晶时间对钒铁结晶分离行为的影响。结果表明,在真空度0.08 MPa, pH值为2.5,硫酸铵添加量(以n((NH4)2SO4)/ n(FeSO4)计)为1.2,反应140 min,结晶36 h的条件下,铁的去除率达86.42%,钒的损失率仅为0.52%,铬的损失率为1.64%,获得了纯度为99.23%的莫尔盐产品。
Abstract:In this study, the leaching solution containing vanadium(IV), chromium(III) and iron(II) obtained in the process of extracting low valence vanadium from vanadium slag was taken as the research object, and the vacuum cooling method was applied to crystallize iron(II) from the leachatein the form of Mohr’s salt, which realized the efficient separation of iron from vanadium(IV) and chromium(III) and the preparation of Mohr’s salt. The effect of pH value, ammonium sulfate addition, reaction time, and crystallization time on the separation behavior of ferrovanadium crystallization were investigated. The results showed that the removal rate of iron reached 86.42% under the conditions of the vacuum degree of 0.08 MPa, pH value of 2.5, addition of ammonium sulfate (calculated as n((NH4)2SO4)/n(FeSO4)) of 1.2, reaction for 140 min and crystallization for 36 h. The loss of vanadium and chromium were only 0.52% and 1.64%, respectively. In addition, Mohr’s salt with a purity of 99.23% was obtained.
-
Key words:
- Vanadium slag /
- Low valence vanadium /
- Crystallize /
- (NH4)2Fe(SO4)2·6H2O
-

-
表 1 浸出液中的主要元素及其含量/(g·t-1)
Table 1. Main chemical compositions in leaching solution
Cr Fe V Ti 1.045 14.890 4.792 0.044 -
[1] 程倩, 王明, 宁新霞, 等. 从某低品位炭质钒矿石中酸浸-萃取-氨沉淀提钒实验研究[J]. 矿产综合利用, 2021(3):17-21.
CHENG Q, WANG M, NING X X, et al. Process of vanadium extraction from a Low-grade carbonaceous vanadium by acid leaching-extraction-ammonia precipitation[J]. Multipurpose Utilization of Mineral Resources, 2021(3):17-21.
[2] 叶国华, 唐悦, 左琪, 等. 叔胺TOA从含钒钢渣直接酸浸液中萃钒除铁的研究[J]. 矿产保护与利用, 2021, 41(3):17-24.
YE G H, TANG Y, ZUO Q, et al. Extracting vanadium over iron from direct acid leaching solution of V-bearing steel slag by solvent extraction with tertiary amine TOA[J]. Conservation and Utilization of Mineral Resources, 2021, 41(3):17-24.
[3] 胡艺博, 叶国华, 左琪, 等. 石煤钒矿酸浸液中萃取提钒的研究进展与前景[J]. 矿产综合利用, 2020(1):10-15. doi: 10.3969/j.issn.1000-6532.2020.01.002
HU Y B, YE G H, ZUO Q, et al. Research progress and prospect of extractants for vanadium from acid leaching solution of stone coal vanadium ore[J]. Multipurpose Utilization of Mineral Resources, 2020(1):10-15. doi: 10.3969/j.issn.1000-6532.2020.01.002
[4] 孙颖, 张廷安, 吕国志, 等. 含钒酸性溶液阴离子萃取分离钒铁的研究[J]. 有色金属(冶炼部分), 2021(4):41-47.
SUN Y, ZHANG T A, LV G Z, et al. Separation of vanadium and iron from vanadium-bearing acidic solution by anion extraction[J]. Nonferrous Metals(Extractive Metallurgy), 2021(4):41-47.
[5] 李丹, 陈德胜, 张国之, 等. 用萃取剂P507从盐酸浸出液中萃取分离钒与铁[J]. 过程工程学报, 2017, 17(6):1182-1187. doi: 10.12034/j.issn.1009-606X.217173
LI D, CHEN D S, ZHANG G Z, et al. Separation of vanadium(IV) from iron(II) in hydrochloric acid solution by solvent extraction with P507[J]. The Chinese Journal of Process Engineering, 2017, 17(6):1182-1187. doi: 10.12034/j.issn.1009-606X.217173
[6] 孟素芬. 利用钛白副产物硫酸亚铁制备电池级磷酸铁的工艺研究[D]. 武汉: 武汉工程大学, 2019.
MENG S F. The Study on preparation of battery grade iron phosphate with titanium dioxide byproduct ferrous sulfate[D]. Wuhan: Wuhan Institute of Technology, 2019.
[7] 邬建辉, 阳伦庄, 湛菁, 等. 铬铁合金中的铬、铁分离研究[J]. 湿法冶金, 2011, 30(1):51-56. doi: 10.3969/j.issn.1009-2617.2011.01.014
WU J H, YANG L Z, ZHAN J, et al. Research on separation of chromium and iron in waste ferrochromium alloy[J]. Hydrometallurgy of China, 2011, 30(1):51-56. doi: 10.3969/j.issn.1009-2617.2011.01.014
[8] 徐敏. 硫酸亚铁铵制备实验的改进[J]. 当代化工, 2015, 44(1):37-38. doi: 10.3969/j.issn.1671-0460.2015.01.012
XU M. Improvement of preparation experiment of ammonium ferrous sulfate[J]. Contemporary Chemical Industry, 2015, 44(1):37-38. doi: 10.3969/j.issn.1671-0460.2015.01.012
[9] 张兆麟, 刘伟. 钛白废酸和副产绿矾生产硫酸亚铁铵[J]. 环境保护, 1993(6):39.
ZHANG Z L, LIU W. Production of ferrous ammonium sulfate from waste titanium dioxide and by-product green vitriol[J]. Environmental Protection, 1993(6):39.
[10] 丁绪淮, 谈遒. 工业结晶[M]. 北京: 化学工业出版社, 1985.
DING X H, TAN Q. Industrial crystallization[M]. Beijing: Chemical Industry Press, 1985.
[11] 钟国清, 吴治先, 白进伟. 硫酸亚铁铵的绿色化制备与表征[J]. 实验室研究与探索, 2015, 34(2):4. doi: 10.3969/j.issn.1006-7167.2015.02.002
ZHONG G Q, WU Z X, BAI J W. Greening preparation and characterization of ammonium ferrous sulfate[J]. Research and Exploration in Laboratory, 2015, 34(2):4. doi: 10.3969/j.issn.1006-7167.2015.02.002
-



 下载:
下载: