Experimental Study on the Removal of Fe2+ and Mn2+ from Zinc Sulfate Water by Micro-nano Ozonation
-
摘要:
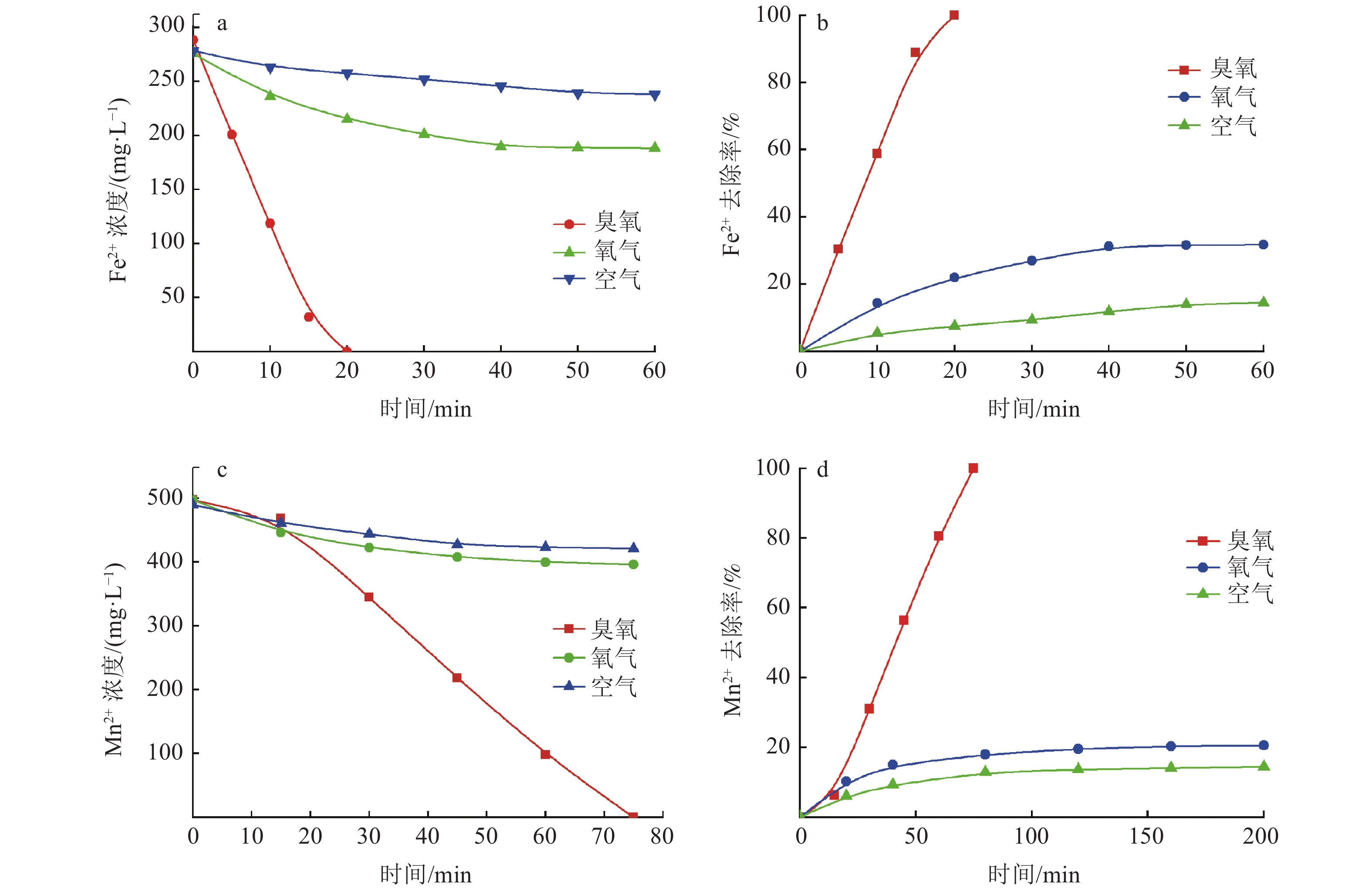
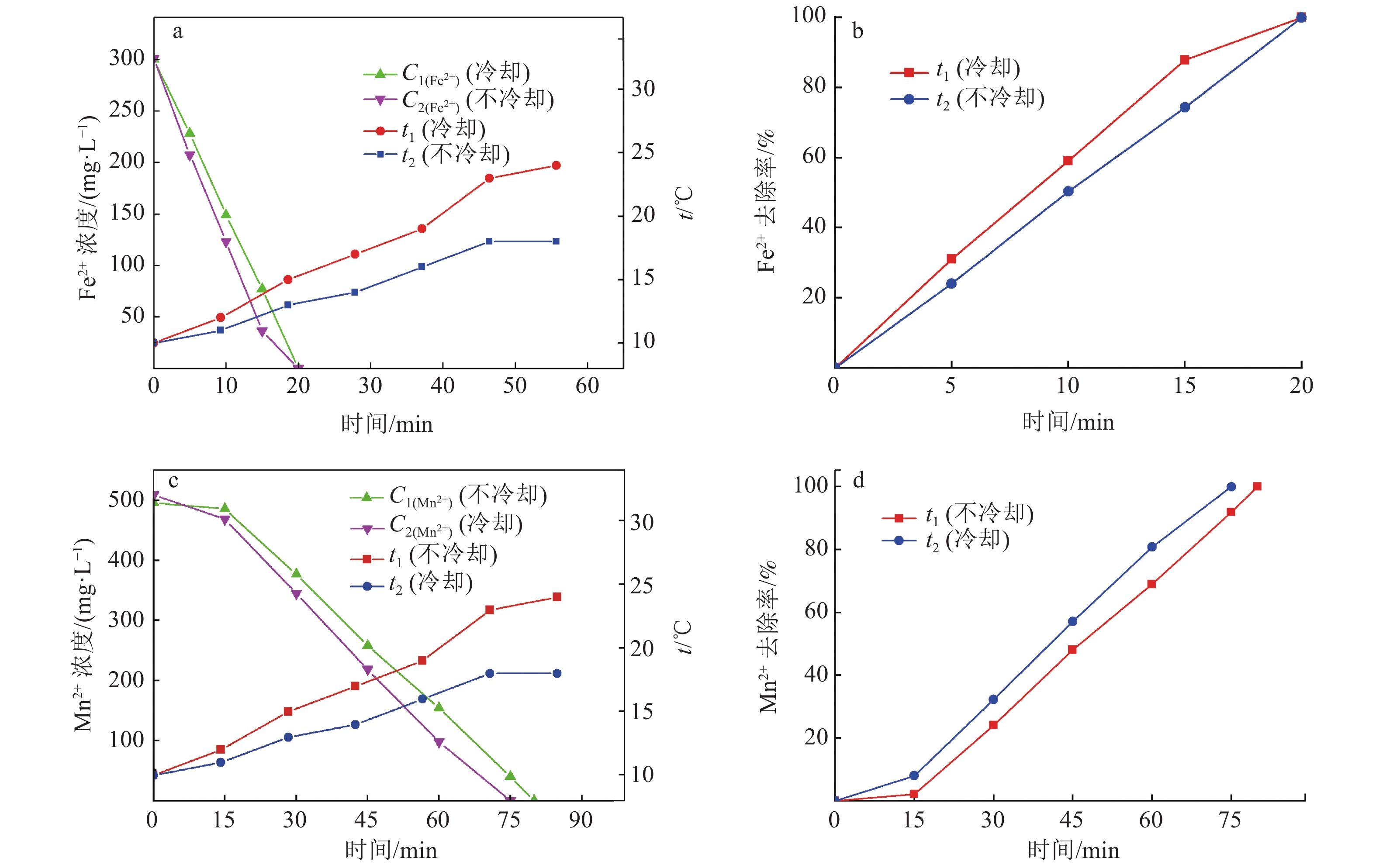
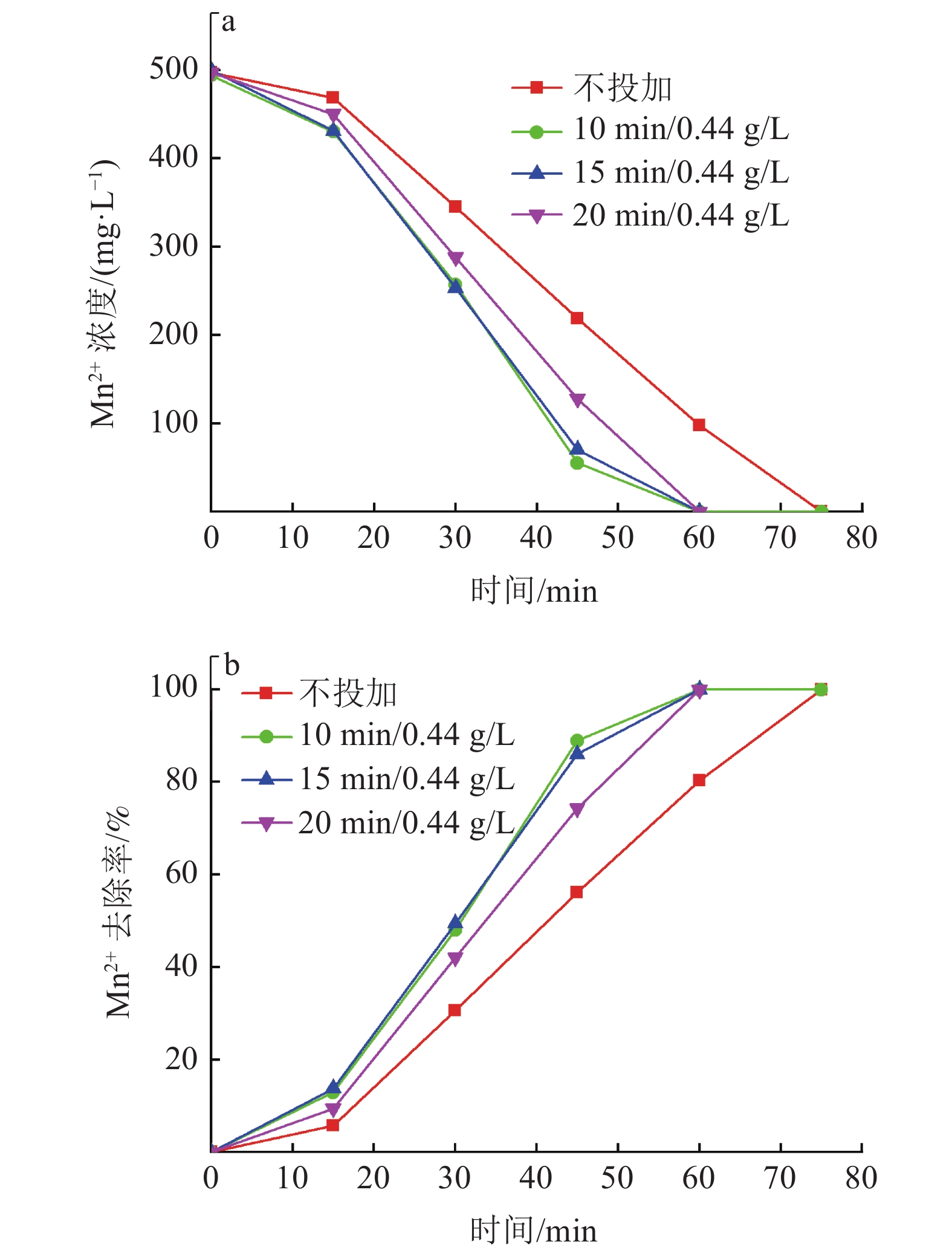
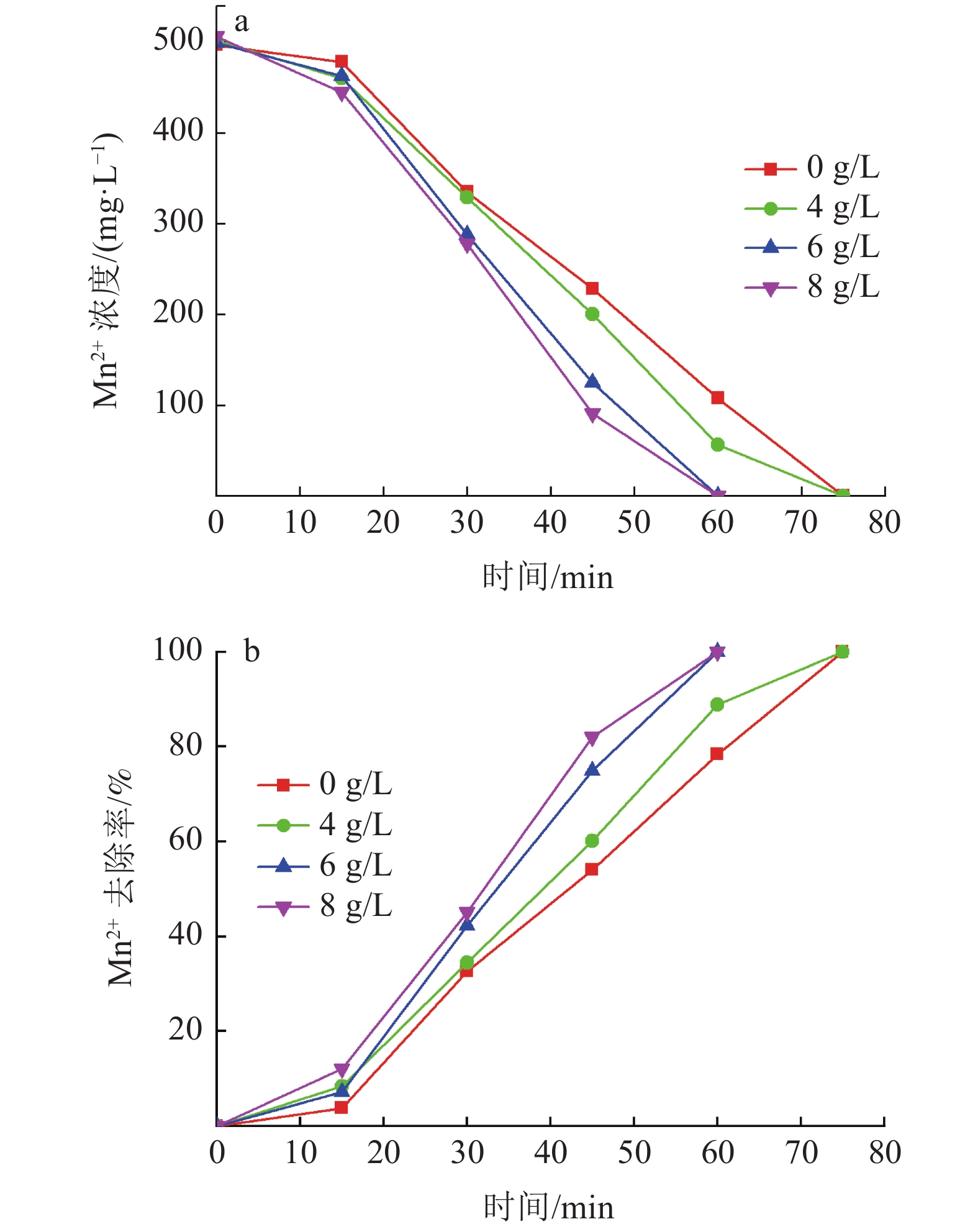
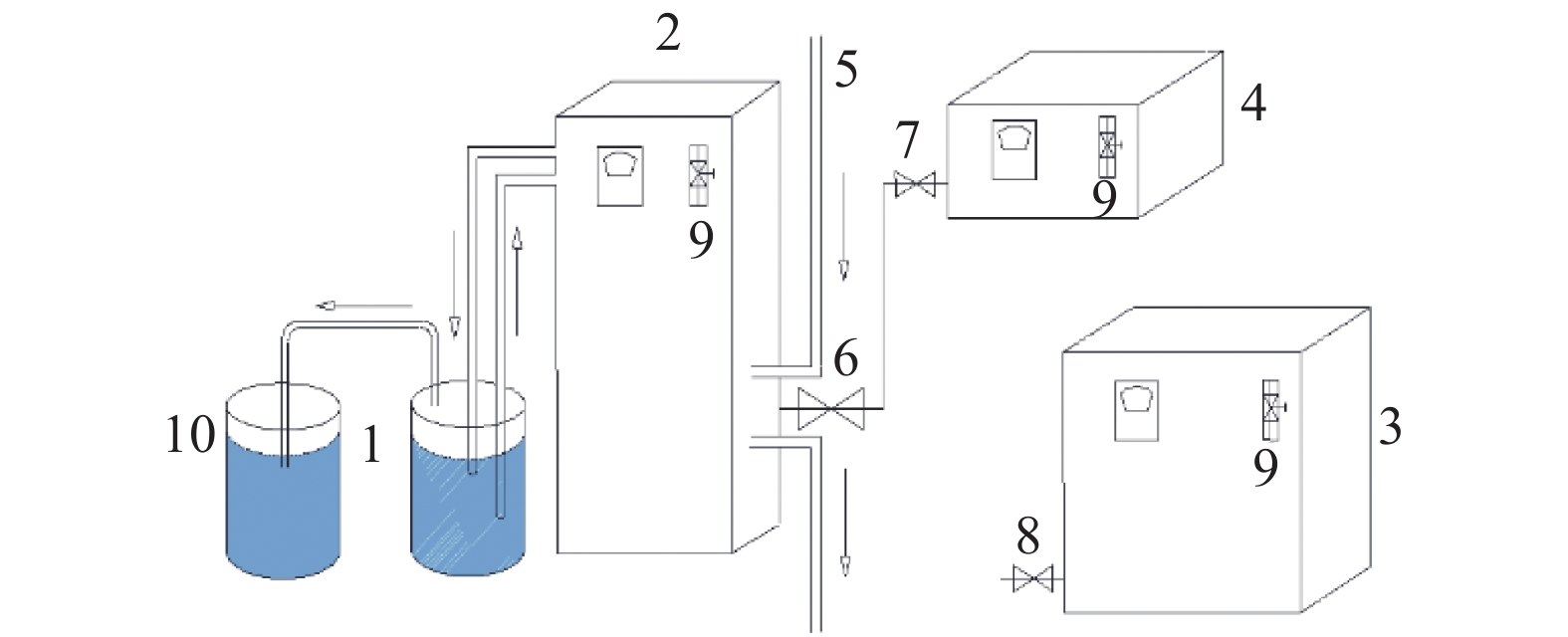
硫酸锌生产过程中,铁锰的存在会对硫酸锌生产结晶工艺造成不利影响,降低MgSO4·7H2O的产品纯度。传统化学处理方法成本较高且易引入新的杂质离子,为获得高质量硫酸锌,开发新的铁锰去除工艺十分重要。本实验采用微纳米气泡和臭氧氧化相结合技术,探究了不同气源、温度、CaCO3投加方式与投加量等条件因素对Fe2+、Mn2+氧化效果的影响,分析该技术在实际生产应用中的可行性。实验结果表明:以臭氧为气源,铁锰去除率达99.5 %,远大于以空气和氧气为气源;温度升高会影响微纳米气泡稳定性与臭氧溶解度,不利于Fe2+、Mn2+的氧化,最高温度18℃时,Mn2+的处理时长相对24℃时缩短了12.5%;CaCO3的投加较大幅度提高了微纳米臭氧除铁锰效率,每15 min以0.44 g/L相对用量投加CaCO3时,CaCO3有效投加总量为17.6 g,铁锰完全去除耗时约50 min,相对于不投加CaCO3时处理时长缩短了33%。间歇式投加效果优于一次性投加,消耗的CaCO3量更少。
Abstract:In the production process of zinc sulfate, the presence of Fe and Mn had an adverse effect on the crystallization process of zinc sulfate production and reduce the purity of MgSO4·7H2O. Traditional chemical treatment methods are costly and easy to introduce new impurity ions. To obtain high-quality zinc sulfate, it is essential to develop a new Fe2+ and Mn2+ removal process. In this experiment, micro-nano bubble and ozone oxidation combined technology was used to explore the influence of different gas sources, temperature, CaCO3 dosage method and dosage on the oxidation effect of Fe2+ and Mn2+, and to analyze the feasibility of the technology in a practical production application. Results showed that the removal rate of Fe2+ and Mn2+ was more than 99.5% with ozone as the gas source, which was much higher than that with air and oxygen as the gas source. The increase of temperature had an effect on the stability of micro and nano bubbles and the solubility of ozone, which was not conducive to the oxidation of Fe2+ and Mn2+. The treatment time of Mn2+ at the maximum temperature of 18℃ was shortened by 12.5% compared with that at 24℃. The addition of CaCO3 significantly improved the removal efficiency of Fe2+ and Mn2+ by micro-nano ozone. When the relative dosage of 0.44 g/L CaCO3 was added every 15 min, the adequate total amount of CaCO3 was 17.6 g, and the complete Removal of Fe2+ and Mn2+ took about 50 min, which shortened the treatment time by 33% compared with the treatment time without CaCO3. Effect of intermittent dosing was better than that of one-time dosing, and the amount of CaCO3 consumed was more petite.
-
Key words:
- Micro-nano bubble /
- Ozonation /
- Iron manganese removal /
- Calcium carbonate
-

-
表 1 原液中主要成分含量
Table 1. Content of main components in the stock solution
成分 Fe2+ Mn2+ Zn2+ Cr3+ 含量/(mg·L-1) 180 530 5×104 50 -
[1] 陈莹博, 王磊, 马洁珺, 等. 氧化法去除废旧锂电池正极材料酸浸液中锰的研究[J]. 有色金属工程, 2020, 10(1):55-61. doi: 10.3969/j.issn.2095-1744.2020.01.009
CHEN Y B, WANG L, MA J J, et al. Study on the removal of manganese in the acid leaching solution of cathode materials of waste lithium batteries by oxidation method[J]. Non-Ferrous Metal Engineering, 2020, 10(1):55-61. doi: 10.3969/j.issn.2095-1744.2020.01.009
[2] Michèle B. Heeb, Kristiana I, Trogolo D, et al. Formation and reactivity of inorganic and organic chloramines and bromamines during oxidative water treatment[J]. Water Research, 2016, 110:91.
[3] 孙国斌, 鄢曙光, 汪小毅, 等. 新型气泡发生器的结构设计和优化模拟研究[J]. 矿产综合利用, 2018(3):144-147+25. doi: 10.3969/j.issn.1000-6532.2018.03.031
SUN G B, YAN S G, WANG X Y, et al. Research on the structure design and optimization simulation of a new type of bubble generator[J]. Multipurpose Utilization of Mineral Resources, 2018(3):144-147+25. doi: 10.3969/j.issn.1000-6532.2018.03.031
[4] LI H Z, HU L M, SONG D J, et al. Subsurface transport behavior of micro-nano bubbles and potential applications for groundwater remediation[J]. International Journal of Environmental Research and Public Health, 2014, 11(1):473-486.
[5] 翟伟哲, 王永刚, 王旭, 等. 微纳米气泡的特性及在水处理技术上的应用研究[J]. 环境科学与管理, 2018(7):95-98. doi: 10.3969/j.issn.1673-1212.2018.07.022
ZHAI W Z, WANG Y G, WANG X, et al. Characteristics of micro-nano bubbles and their application in water treatment technology[J]. Environmental Science and Management, 2018(7):95-98. doi: 10.3969/j.issn.1673-1212.2018.07.022
[6] 熊永磊, 杨小丽, 宋海亮. 微纳米气泡在水处理中的应用及其发生装置研究[J]. 环境工程, 2016(6):23-27. doi: 10.13205/j.hjgc.201606006
XIONG Y L, YANG X L, SONG H L. Application of micro-nano bubbles in water treatment and research on its generating device[J]. Environmental Engineering, 2016(6):23-27. doi: 10.13205/j.hjgc.201606006
[7] 杨丽, 陈海军, 杨谋存, 等. 微纳米气泡发生装置及其应用的研究进展[J]. 石油化工, 2014(10):1206-1213. doi: 10.3969/j.issn.1000-8144.2014.10.018
YANG L, CHEN H J, YANG M C, et al. Progresses in research and application of micro-nano bubble generating device[J]. Petrochemical Technology, 2014(10):1206-1213. doi: 10.3969/j.issn.1000-8144.2014.10.018
[8] 温舒涵, 姚沁坪, 李炜琦, 等. MnO2陶粒臭氧氧化催化剂的制备及其性能[J]. 化工环保, 2018(2):157-163. doi: 10.3969/j.issn.1006-1878.2018.02.006
WEN S H, YAO Q P, LI W Q, et al. Preparation and performance of MnO2 ceramsite ozone oxidation catalyst[J]. Environmental Protection of Chemical Industry, 2018(2):157-163. doi: 10.3969/j.issn.1006-1878.2018.02.006
[9] 徐璐, 何兰军, 杨耀辉, 等. 从云南某锌浸出渣中回收锌锗的试验研究[J]. 矿产综合利用, 2020(1):116-119+75. doi: 10.3969/j.issn.1000-6532.2020.01.024
XU L, HE L J, YANG Y H, et al. Experimental study on recovery of zinc and germanium from a zinc leaching residue in Yunnan[J]. Multipurpose Utilization of Mineral Resources, 2020(1):116-119+75. doi: 10.3969/j.issn.1000-6532.2020.01.024
[10] 刘天明, 王頔, 胡光楠, 等. 生物法处理含铁锰地下水的研究进展[J]. 辽宁化工, 2015(7):911-914. doi: 10.14029/j.cnki.issn1004-0935.2015.07.049
LIU T M, WANG D, HU G N, et al. Research progress of biological treatment of groundwater containing iron and manganese[J]. Liaoning Chemical Industry, 2015(7):911-914. doi: 10.14029/j.cnki.issn1004-0935.2015.07.049
[11] 李圭白, 梁恒, 余华荣, 等. 锰质活性滤膜化学催化氧化除锰机理研究[J]. 给水排水, 2019(5):6-10+75. doi: 10.13789/j.cnki.wwe1964.2019.05.001
LI G B, LIANG H, YU H R, et al. Study on the mechanism of manganese active filter membrane chemical catalytic oxidation to remove manganese[J]. Water & Wastewater, 2019(5):6-10+75. doi: 10.13789/j.cnki.wwe1964.2019.05.001
[12] 黄磊, 唐琪玮, 黄思远, 等. 臭氧氧化技术及其在水处理领域的发展[J]. 净水技术, 2018, 37(S1):106-112. doi: 10.15890/j.cnki.jsjs.2018.s1.028
HUANG L, TANG Q W, HUANG S Y, et al. Ozone oxidation technology and its development in the field of water treatment[J]. Water Purification Technology, 2018, 37(S1):106-112. doi: 10.15890/j.cnki.jsjs.2018.s1.028
[13] 常佳伟, 樊金梦, 王伟, 等. 旋转填充床中臭氧氧化处理兰炭废水生化出水[J]. 化工环保, 2020(2):131-136. doi: 10.3969/j.issn.1006-1878.2020.02.004
CHANG J W, FAN J M, WANG W, et al. Ozone oxidation treatment of biochemical effluent from blue charcoal wastewater in a rotating packed bed[J]. Environmental Protection of Chemical Industry, 2020(2):131-136. doi: 10.3969/j.issn.1006-1878.2020.02.004
-



 下载:
下载: