Study on the Factors Influencing on Potassium Feldspar - Phosphogypsum - Activated Carbon Potassium Extraction Process
-
摘要:
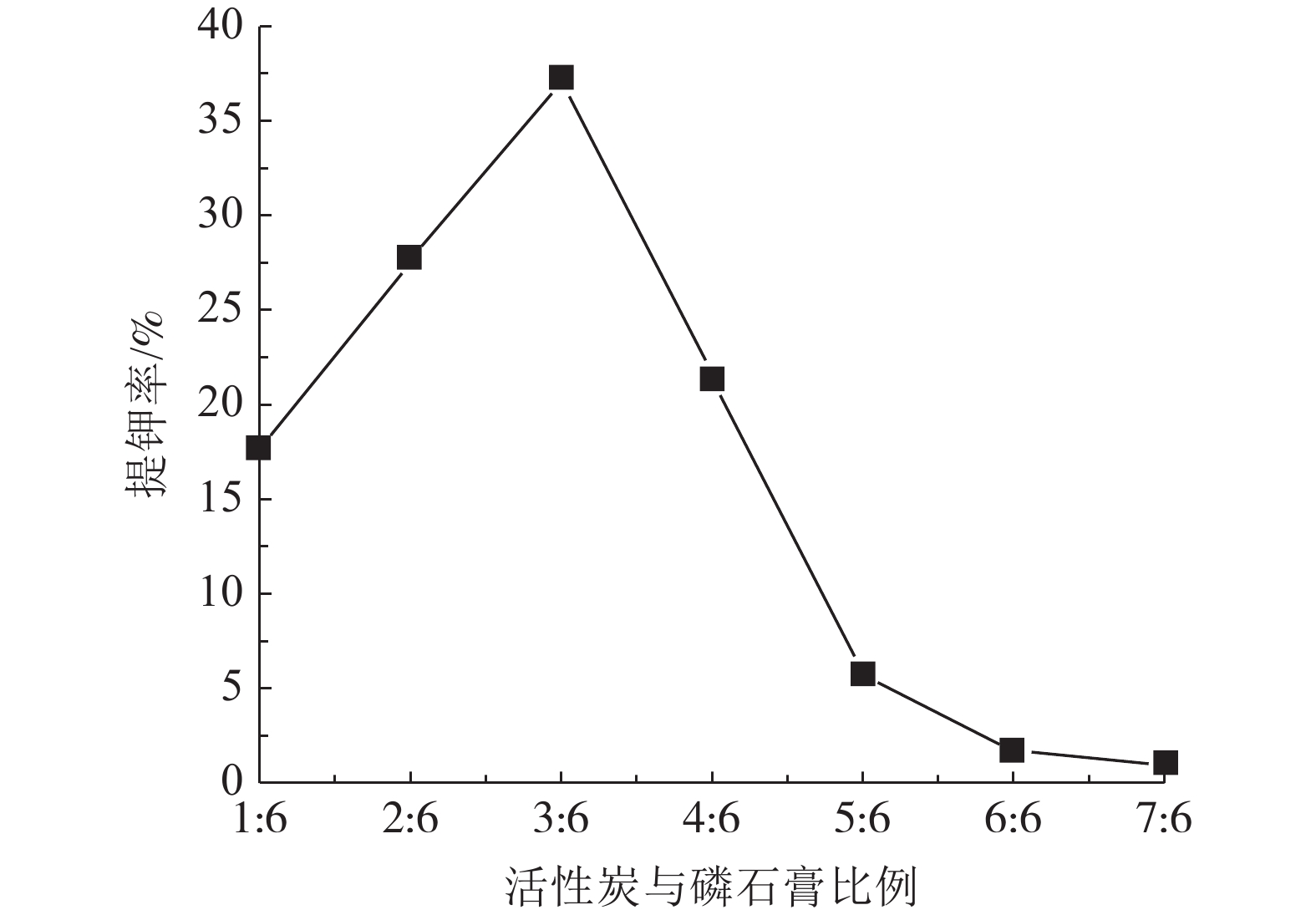
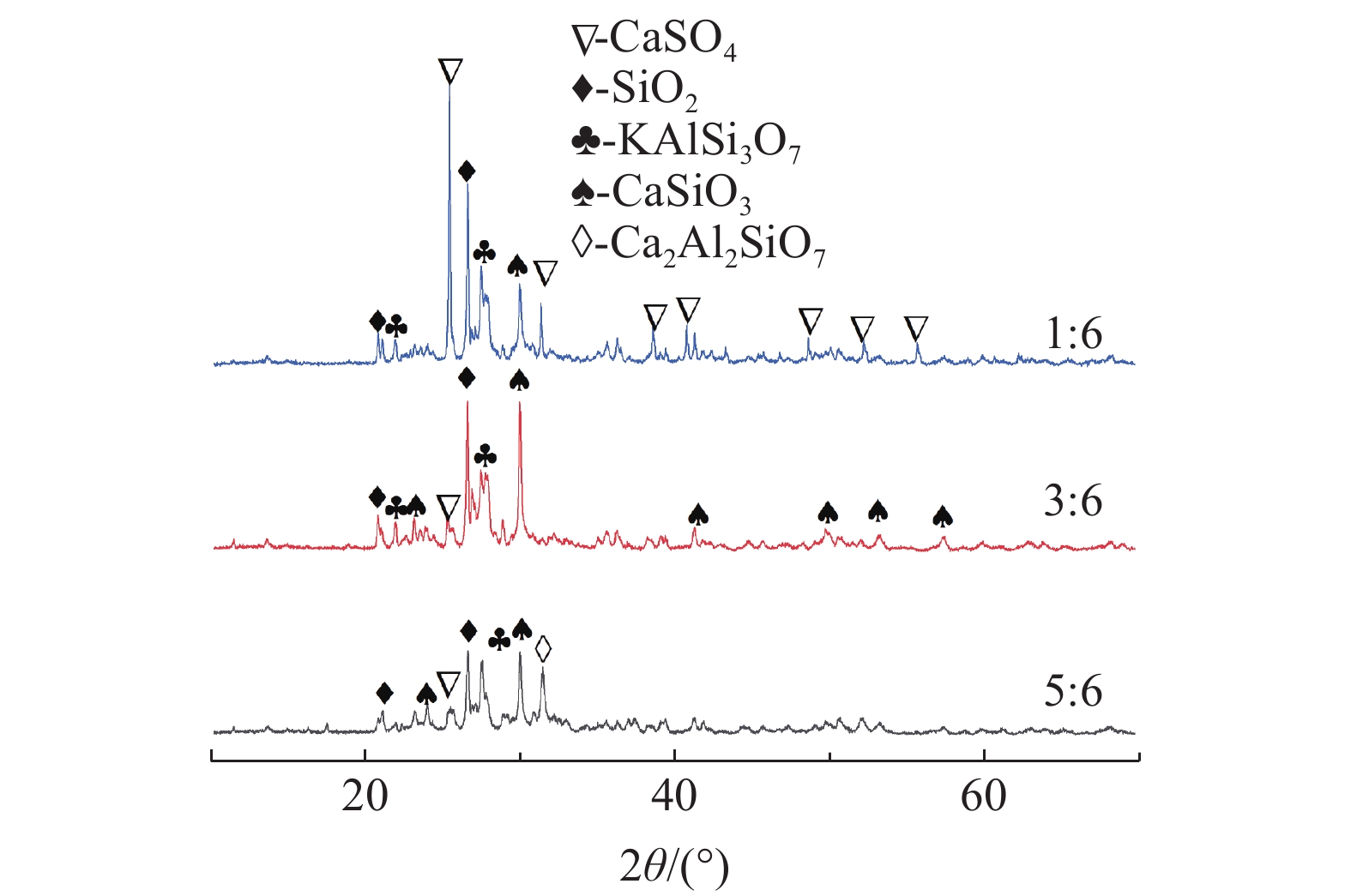
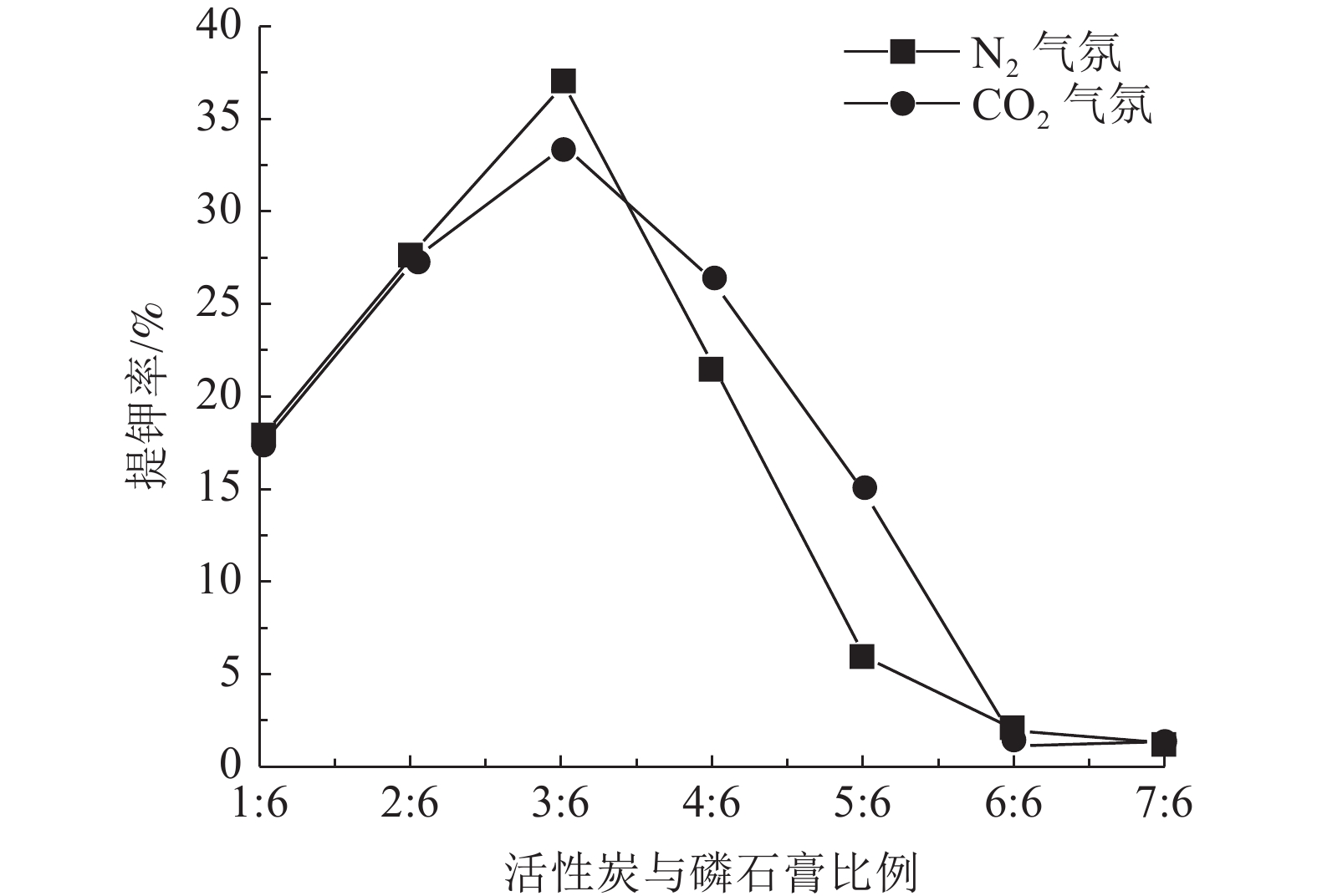
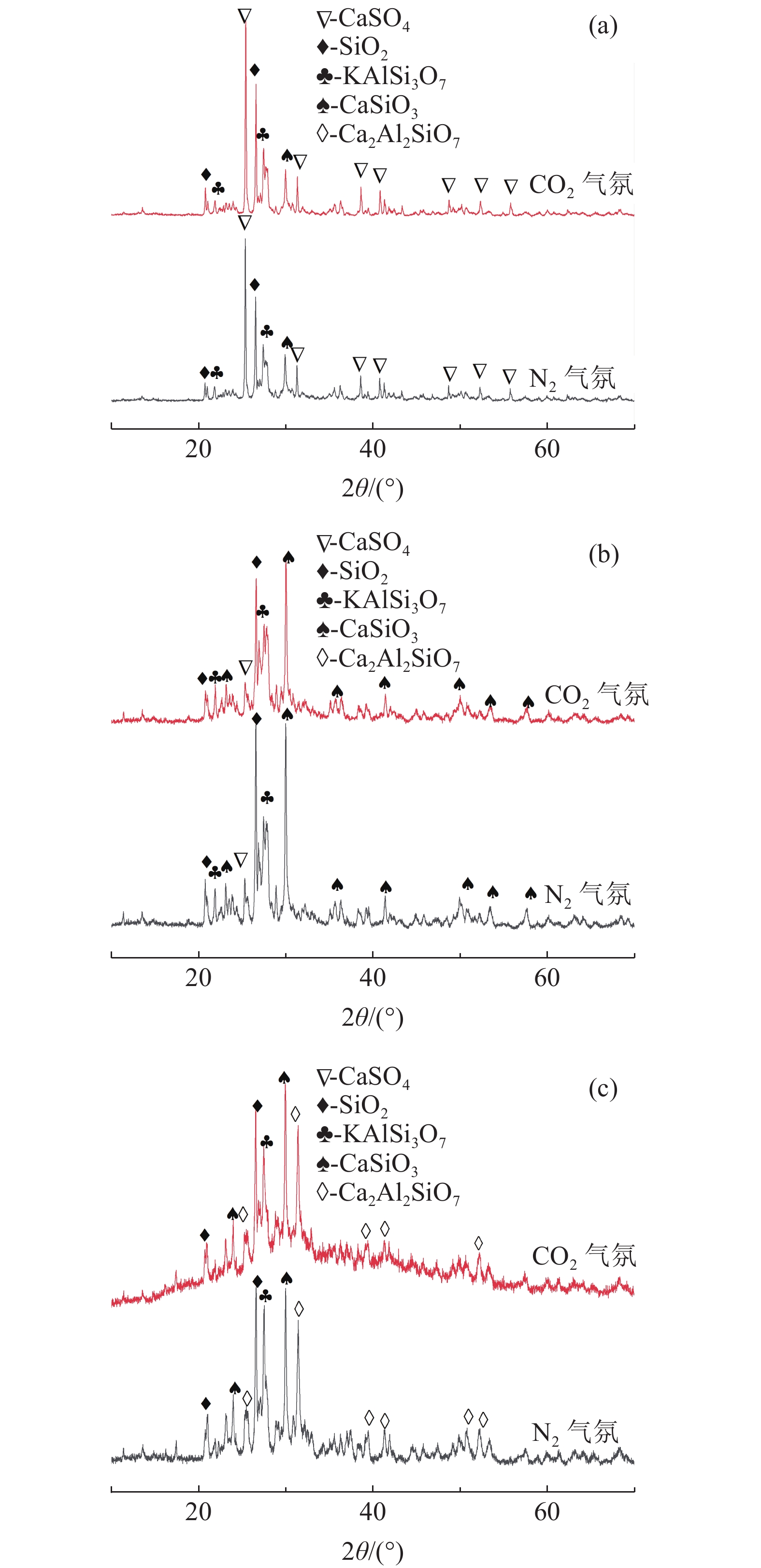
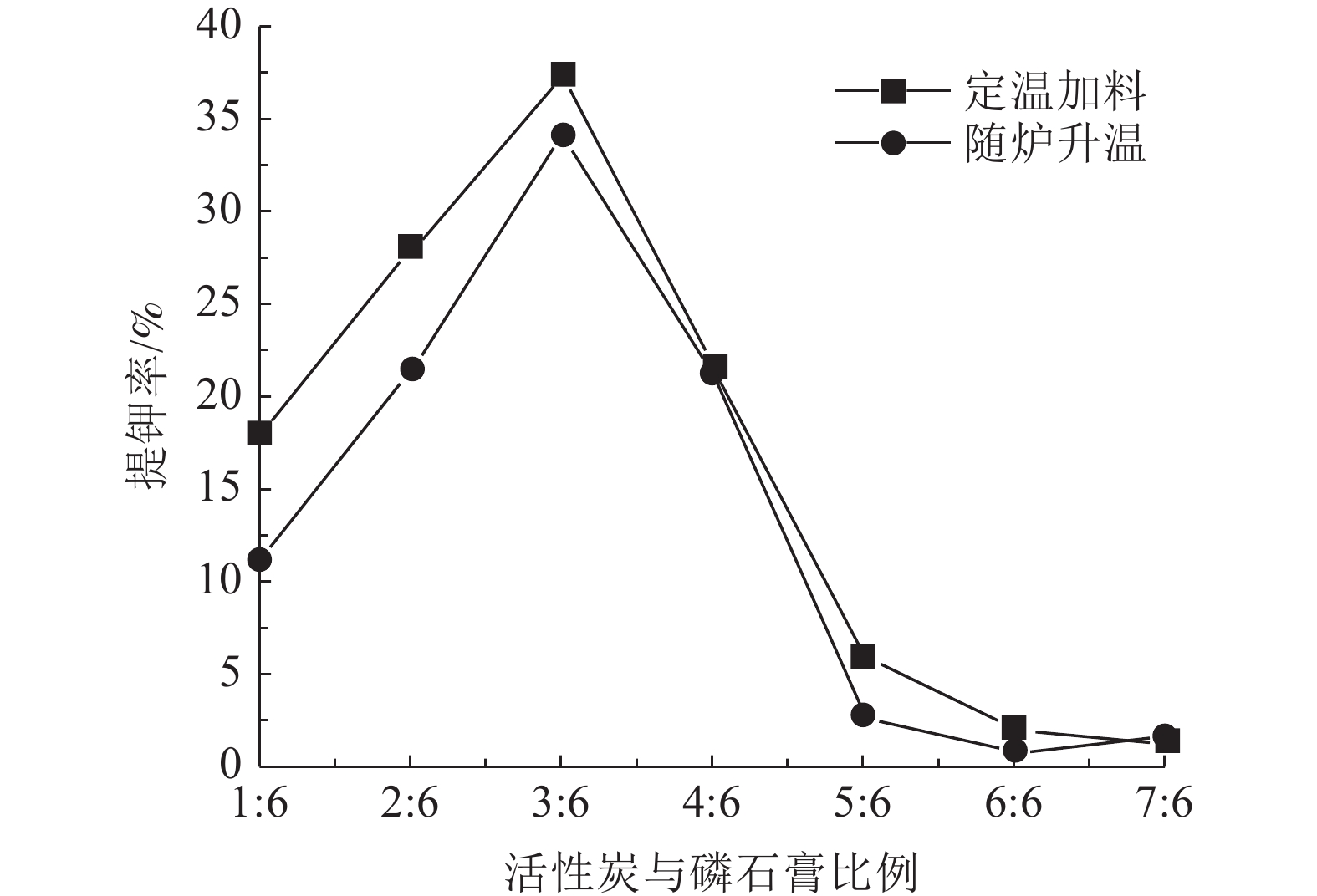
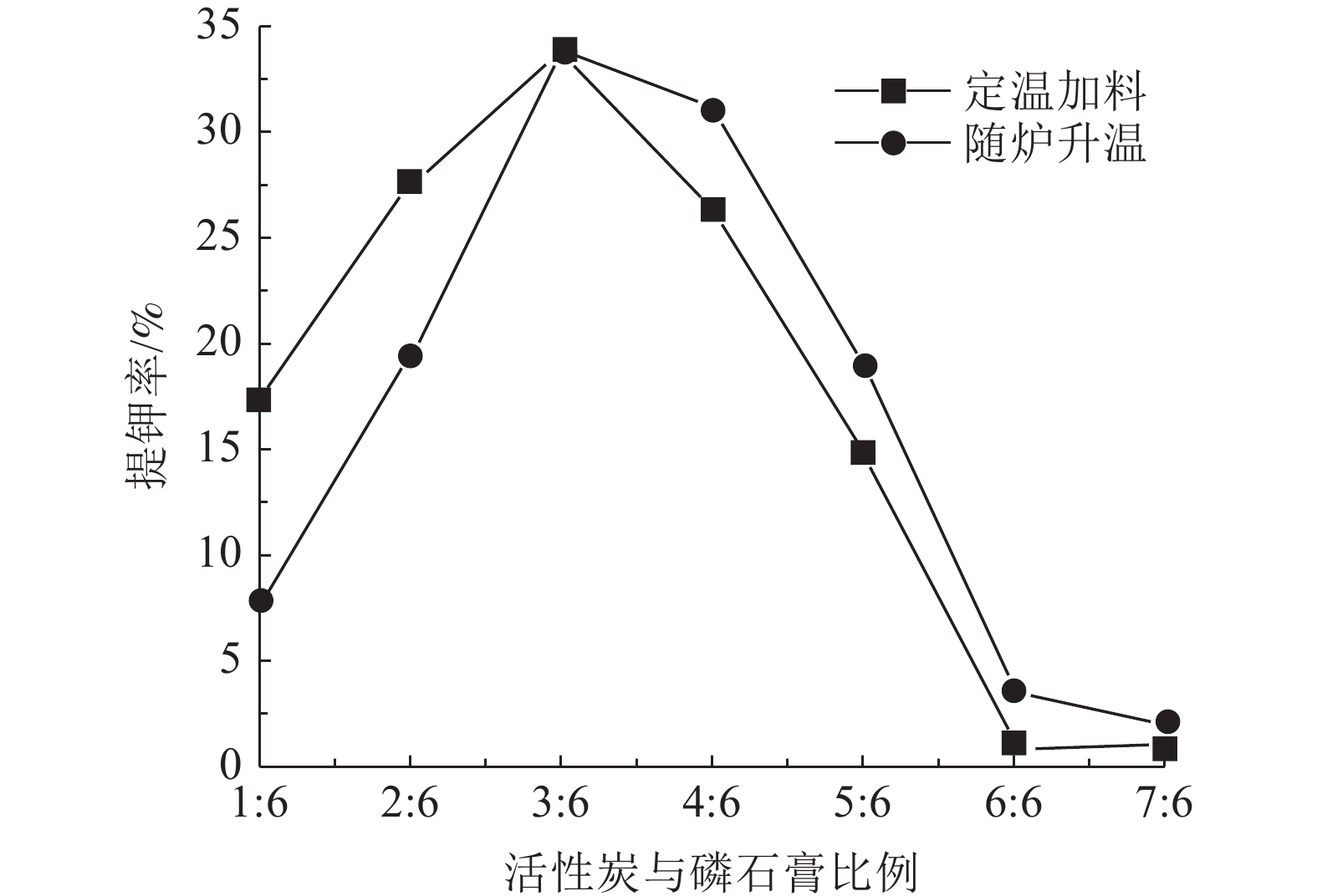
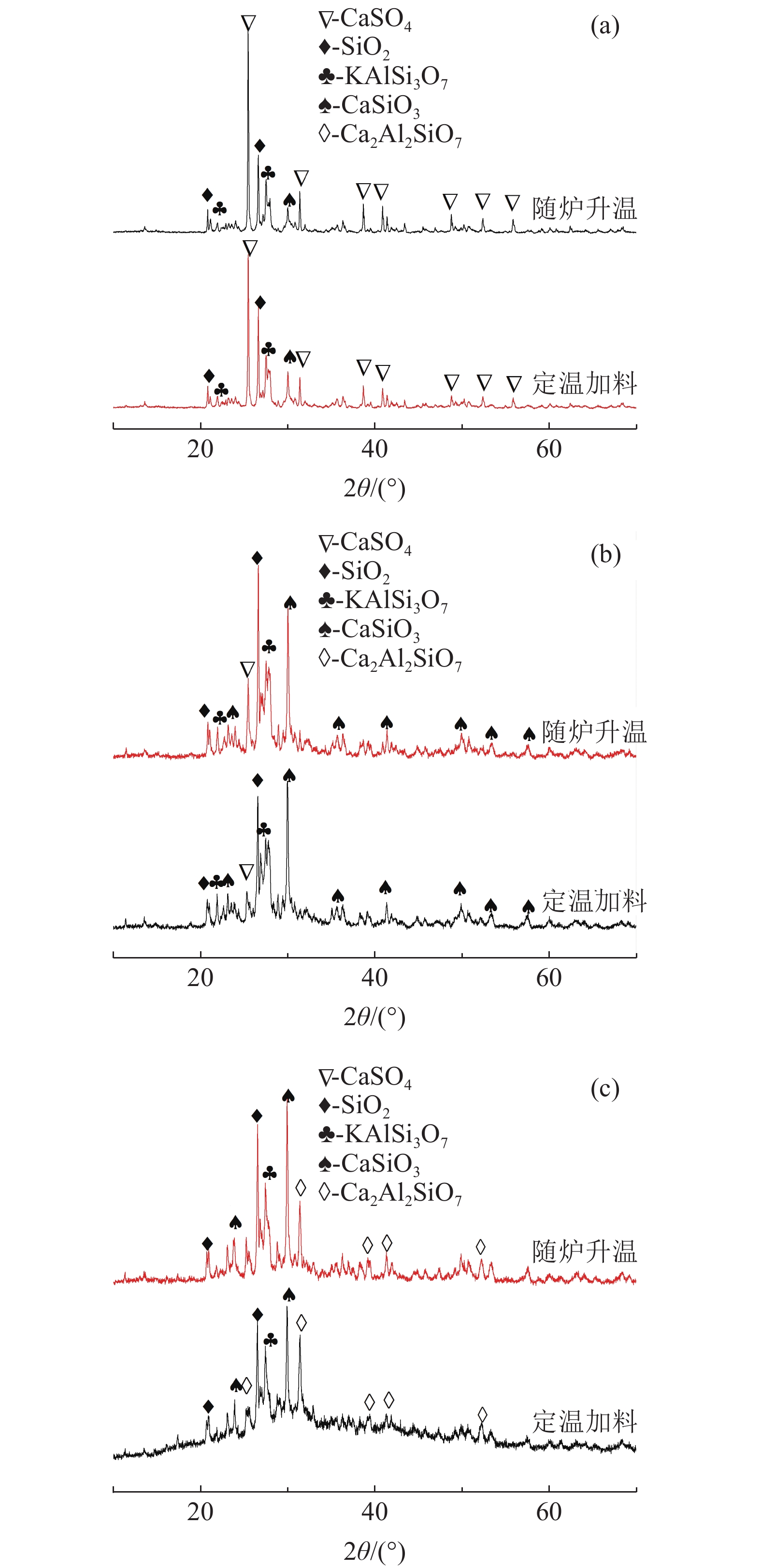
钾长石-磷石膏-焦炭提取钾肥是利用非可溶性钾资源的可行工业化路线,其中焦炭与磷石膏的反应过程是影响提钾率的关键,本文研究了N2或CO2气氛下活性炭比例对体系提钾率的影响,并采用了随炉升温与定温加料两种不同的焙烧温度制度对比了不同气氛下的提钾率和焙烧产物。结果表明,随着活性炭在体系中含量的升高,提钾率出现先上升后下降的趋势。在N2气氛下,采用定温加料体系的提钾率大于随炉升温的提钾率;在CO2气氛下,当活性炭与磷石膏的比例小于3∶6,定温加料样品的提钾率大于随炉升温的样品,而活性炭与磷石膏的比例大于3∶6,随炉升温样品的提钾率大于定温加料样品。
Abstract:Extracting process of potassium fertilizer with potassium feldspar-phosphogypsum-coke system is a feasible industrialization route to utilize insoluble potassium resources, in which the reaction process of coke and phosphogypsum is the key to raise the potassium extraction rate. In this paper, the influence of the ratio of activated carbon to phosphogypsum on potassium extraction rate is studied under N2 or CO2 atmosphere. Two different roasting temperature systems, heating up with the furnace and feeding at constant temperature, are used to compare the potassium extraction rate and roasted products under different atmospheres. The results showed that as the content of activated carbon in the system increased, the potassium extraction rate increased first and then decreased. Under N2 atmosphere, the rate of potassium extraction in samples that heating with constant temperature is greater than that of samples heating with the furnace. In CO2 atmosphere, when the ratio of activated carbon to phosphogypsum is less than 3:6, the potassium extraction rate of samples heating with constant temperature is greater than that of samples heating with the furnace; when the ratio of activated carbon to phosphogypsum is greater than 3∶6, the potassium extraction rate of samples heating with the furnace is greater than that of samples heating with constant temperature.
-

-
表 1 钾长石与磷石膏的主要成分/%
Table 1. Chemical compositions of feldspar and phosphogypsum
名称 SiO2 Al2O3 Fe2O3 CaO K2O Na2O SO3 MgO P2O5 钾长石 65.83 17.18 2.05 2.04 7.28 2.05 0 0.51 0 磷石膏 4.51 0 0.13 39.98 0 0 54.11 0.21 1.29 -
[1] 李文学, 孙明光. 尾盐中Na+、K+、SO4 2−、Mg2+、Cl− 离子的分布特点及能再利用钾硫资源量概算[J]. 矿产综合利用, 2019(4):111-116. doi: 10.3969/j.issn.1000-6532.2019.04.024
LI W X, SUN M G. The Distribution Characteristics of Na+, K+, SO4 2−, Mg2+and Cl− in tail salt and the estimate of the recoverable potassium and sulfur resource[J]. Multipurpose Utilization of Mineral Resources, 2019(4):111-116. doi: 10.3969/j.issn.1000-6532.2019.04.024
[2] 亓昭英, 屈小荣, 杜双江, 等. 2019年我国钾肥行业运行情况及未来5年发展趋势分析[J]. 磷肥与复肥, 2020, 35(4):1-5. doi: 10.3969/j.issn.1007-6220.2020.04.002
QI Z Y, QU X R, DU S J, et al. Analysis on the operation of potash fertilizer industry in 2019 and its development trend in the next five years[J]. Phosphate & Compound Fertilizer, 2020, 35(4):1-5. doi: 10.3969/j.issn.1007-6220.2020.04.002
[3] 胡波, 韩效钊, 肖正辉, 等. 我国钾长石矿产资源分布、开发利用、问题与对策[J]. 化工矿产地质, 2005(1):25-32. doi: 10.3969/j.issn.1006-5296.2005.01.005
HU B, HAN X Z, XIAO Z H, et al. Distribution of potash feldspar resources in China and its exploitation[J]. Chemical Minerals Geology, 2005(1):25-32. doi: 10.3969/j.issn.1006-5296.2005.01.005
[4] 宋一涵, 李洪枚, 马淑花, 等. 钾长石提钾及制备复合肥料的研究进展[J]. 过程工程报, 2005, 18(1):25-32.
SONG Y H, LI H M, MA S H, et al. Research progress on extracting potassium and preparing compound fertilizer from potassium feldspar[J]. Chinese Journal of Process Engineering, 2005, 18(1):25-32.
[5] 陈定盛, 石林, 汪碧容, 等. 焙烧钾长石制硫酸钾的实验研究[J]. 化肥工业, 2006(6):20-23. doi: 10.3969/j.issn.1006-7779.2006.06.006
CHEN D S, SHI L, WANG B R, et al. Experimental study of calcination of potash feldspar for manufacture of potassium sulfate[J]. Chemical Fertilizer Industry, 2006(6):20-23. doi: 10.3969/j.issn.1006-7779.2006.06.006
[6] 李兴平, 刘阳, 张西兴, 等. 磷石膏、钾长石与焦炭热反应试验及机理研究[J]. 矿产综合利用, 2017(5):124-128+123. doi: 10.3969/j.issn.1000-6532.2017.05.028
LI X P, LIU Y, ZHANG X X, et al. Study on the thermal reaction test and mechanism of phosphorus gypsum, potassium feldspar and coke[J]. Multipurpose Utilization of Mineral Resources, 2017(5):124-128+123. doi: 10.3969/j.issn.1000-6532.2017.05.028
[7] 李兴平, 刘阳, 胡兆平. 石灰石和钾长石焙烧法制备硅钙钾肥试验研究[J]. 矿产综合利用, 2020(2):82-86. doi: 10.3969/j.issn.1000-6532.2020.02.014
LI X P, LIU Y, HU Z P. Study on preparation of silicon-calcium-potassium fertilizer by calcining limestone and potassium feldspar[J]. Multipurpose Utilization of Mineral Resources, 2020(2):82-86. doi: 10.3969/j.issn.1000-6532.2020.02.014
[8] 任雪娇. 石膏、钾长石热反应基础研究[D]. 昆明: 昆明理工大学, 2013.
REN X J. Basic study on thermal reaction of gypsum and potassium feldspar[D]. Kunming: Kunming University of Science and Technology, 2013.
[9] 夏举佩, 彭健, 李国斌, 等. 钾长石在CaSO4及其分解产物下的焙烧反应研究[J]. 非金属矿, 2014, 37(5):14-17. doi: 10.3969/j.issn.1000-8098.2014.05.005
XIA J P, PENG J, LI G B, et al. Calcination reaction of potassium feldspar in CaSO4 and its decomposition products[J]. Non-Metallic Mines, 2014, 37(5):14-17. doi: 10.3969/j.issn.1000-8098.2014.05.005
[10] 徐仁伟. 焦炭及其杂质对硫酸钙热解过程影响的研究[D]. 上海: 华东理工大学, 2011.
XU R W. Study on the influence of coke and its impurities on pyrolysis process of calcium sulfate[D]. Shanghai: East China University Of Science and Technology, 2011.
[11] 李晓亚, 周托, 那永洁, 等. CaS氧化和CaS与CaSO4固固反应机理[J]. 中国粉体技术, 2019, 25(2):18-24.
LI X Y, ZHOU T, NA Y J, et al. Mechanism of oxidation reaction of CaS and solid-solid reaction of CaS and CaSO4[J]. China Powder Science and Technology, 2019, 25(2):18-24.
[12] 任雪娇, 夏举佩, 张召述. 磷石膏还原分解反应热力学分析[J]. 环境工程学报, 2013, 7(3):1128-1132.
REN X J, XIA J P, ZHANG Z S. Thermodynamic analysis of reductive decomposition for phosphogypsum[J]. Chinese Journal of Environmental Engineering, 2013, 7(3):1128-1132.
[13] 燕春培, 郁青春, 刘大春, 等. 真空碳热还原分解硫酸钙热力学分析及实验探究[J]. 真空科学与技术学报, 2014, 34(5): 517-521.
YAN C P, YU Q C, LIU D C, et al. Decomposition of calcium sulphate by carbothermic reduction at reduced pressures [J]. Chinese Journal of Vacuum Science and Technology, 2014, 3 4(5): 517-521.
-



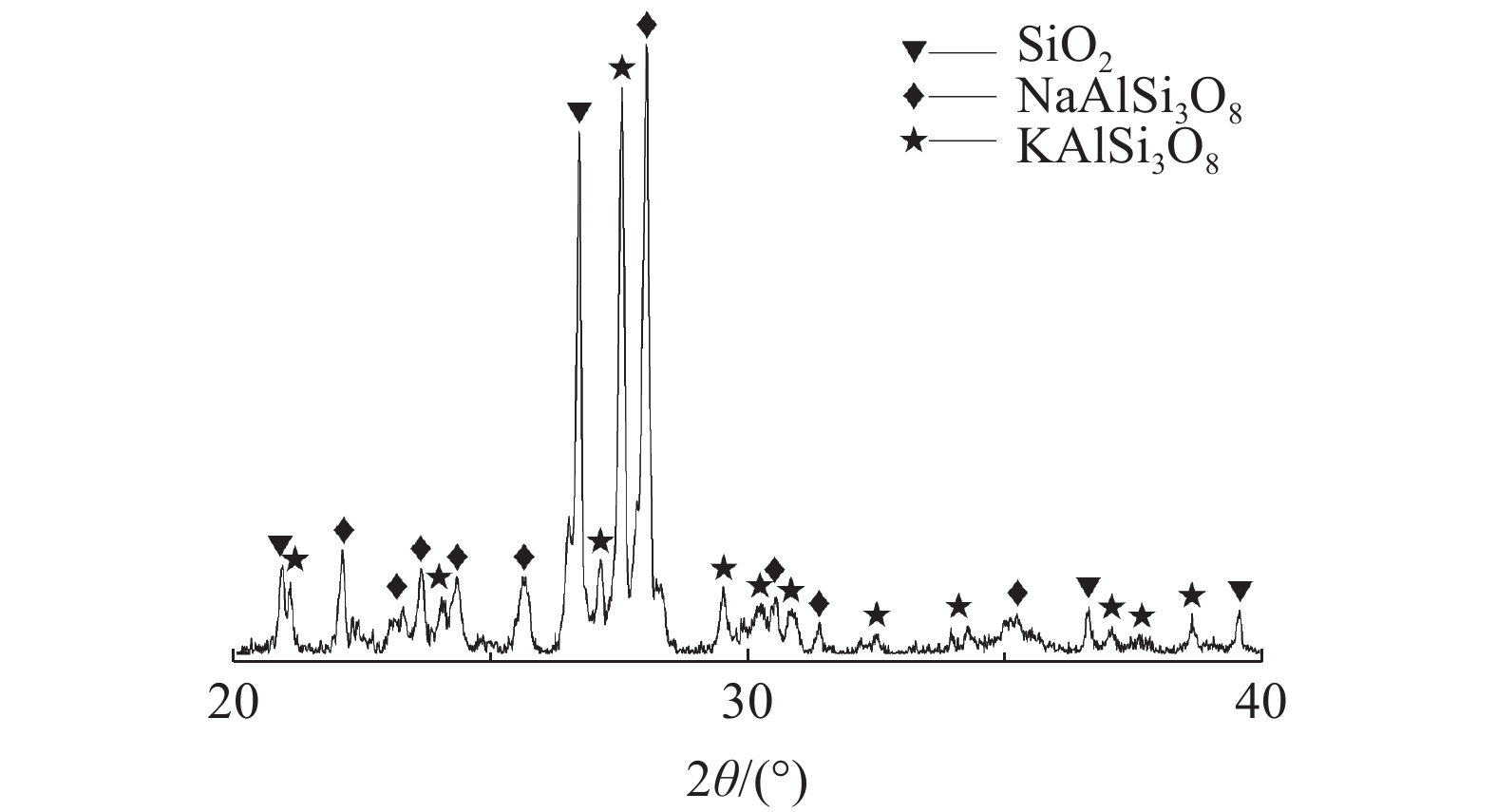
 下载:
下载: