Rutile Flotation properities and its Mechanism in Synergistic Systems Composed by Sodium Oleate and Hydroximic Acid-type Reagents Bearing Benzene Ring
-
摘要:
为揭示油酸钠(SO)和羟肟酸类药剂协同浮选金红石的协同机理,在单矿物浮选的基础上,采用Zeta电位、表面张力、接触角、紫外漫反射光谱等手段从界面化学角度探索了组合药剂在金红石表面的作用行为。结果表明:三种药剂对金红石捕收能力大小顺序为:油酸钠(SO)>苯甲羟肟酸钠(BHA)>水杨羟肟酸钠(SHA)。药剂添加顺序对组合体系中金红石的浮选行为影响较大,油酸钠分子中富电子的双键与羟肟酸类药剂分子中缺电子的苯环之间具有电子共轭效应,该效应可引起组合药剂发生缔合并生成缔合物从而对金红石产生协同作用。苯甲羟肟酸钠与油酸钠组合体系中金红石浮选回收率与药剂作用后矿物的带隙大小存正比例关系,而在水杨羟肟酸钠和油酸钠组合体系中则成反比例关系。
Abstract:Flotation behaviors of rutile in mixed reagent systems of sodium oleate (SO)/sodium benzohydroxamide (BHA) and sodium oleate (SO)/sodium salicylhydroxamide (SHA) had been investigated. The interaction mechanisms between reagents as well as the adsorption properties of reagents onto rutile surface were studied using several methods, namely Zeta potential, surface intension, contact angle, UV diffuse reflectance spectroscopy et al. The results indicate that the collecting ability of the three reagents for rutile follows the order: SO>BHA>SHA. The adding sequence of reagents is of great importance to rutile flotation properties, eg., the rutile recovery and the synergistic effect index when adding BHA or SHA firstly and sodium oleate secondly are higher than the rest situations. There is an electron conjugation effect between the electron-rich double bond in sodium oleate and the benzene ring in SHA or BHA which is the motivation for reagents association. The amount and configuration of the association complex play an critical role in rutile flotation nature. It is observed that the rutile recovery tally with the width of rutile in SO/BHA system while in SO/SHA system the relationship is reversed and the reasons need to be further studied.
-
Key words:
- Rutile flotation /
- Mixed reagents /
- Synergistic effect /
- Rutile flotation
-

-
表 1 不同体系中组合药剂的的协同效应指数
Table 1. Synergism values of different combined reagents for rutile flotation
油酸钠质量含量/% 协同效应指数 后加入油酸钠 预先混合 先加入油酸钠 先加入苯甲羟
肟酸钠先加入水杨羟
肟酸钠油酸钠与苯甲羟
肟酸钠油酸钠与水杨羟
肟酸钠后加入苯甲羟
肟酸钠后添加入杨羟
肟酸钠0 0.00 0.00 0.00 0.00 0.00 0.00 10 0.90 1.55 0.60 0.29 0.40 1.20 20 0.91 1.22 0.73 0.29 0.59 0.78 30 1.17 1.16 0.97 0.31 0.72 0.48 40 1.07 0.91 0.94 0.12 0.83 0.26 50 1.08 0.73 0.97 0.01 1.01 0.15 60 1.06 0.89 0.95 0.06 0.69 0.12 70 0.89 1.00 0.80 0.00 0.43 0.10 80 0.87 0.80 0.70 0.15 0.28 0.22 90 1.38 0.54 1.02 0.35 0.49 0.38 100 0.00 0.00 0.00 0.00 0.00 0.00 表 2 不同体系中金红石浮选回收率与药剂比例的拟合曲线
Table 2. Curving fittings for rutile recovery against reagent ratioa in various systems
试剂体系 拟合方程 R2 SD P值 预先混合 油酸钠和苯甲羟肟酸钠 ε=38.46+2.91n-0.08n2+9.7×10-4n3-4.9×10-6n4 0.98 2.85 <10-4 油酸钠和水杨羟肟酸钠 ε=4.74+0.53n 0.97 2.65 <10-4 先添加油酸钠 后添加苯甲羟肟酸钠 ε=36.69+2.11n-0.03n2+1.13×10-4n3 0.93 4.03 <10-4 后添加水杨羟肟酸钠 ε=9.36+0.50n 0.95 2.80 <10-4 后添加油酸钠 先添加苯甲羟肟酸钠 ε=38.87+4.45n-0.15n2+0.002n3-1.03×10-5n4 0.94 2.43 <10-4 先添加水杨羟肟酸钠 ε=3.33+2.44n-0.08n2+0.001n3-7.75×10-6 n4 0.99 2.27 <10-4 注:ε=100×(金红石回收率),n=100×(油酸钠质量含量) -
[1] 和平志, 黄淑梅, 郭薇, 等. 世界海绵钛工业的现状及对我国未来发展的思考[J]. 钛工业进展, 2017, 34(4):1-4.
HE P Z, HUANG S M, GUO W, et al. Present aituation of world titanium sponge industry and future development in China[J]. Titanium Industry Progress, 2017, 34(4):1-4.
[2] LIU Q, PENG Yongjun. The development of a composite collector for the flotation of rutile[J]. Minerals Engineering, 1999, 12(12):1419-1430.
[3] 吕宪俊, 高清寿. 陕西安康金红石矿工艺矿物学研究[J]. 矿产保护与利用, 1999(6):37-40.
LV X J, GAO Q S. A study on the process mineralogy of rutile ore from Ankang, Shaanxi[J]. Conservation and Utilization of Mineral Resourses, 1999(6):37-40.
[4] 朱建光. 浮选金红石用的捕收剂和调整剂[J]. 国外金属矿选矿, 2008(2):3-8.
ZHU J G. Collector and regulator for rutile flotation[J]. Metallic Ore Dressing Abord, 2008(2):3-8.
[5] 华中宝, 童雄, 谢贤, 等. 金红石浮选药剂研究进展[J]. 金属矿山, 2018(9):28-32.
HUA Z B, TONG X, XIE X, et al. Review of rutile flotation reagents and their mechanisms[J]. Metal Mine, 2018(9):28-32.
[6] 张闿. 浮选药剂的组合使用[M]. 北京: 冶金工业出版社, 1994: 58-61.
ZHANG K. The combined use of flotation reagents [M]. Beijing: Metallurgical Industry Press, 1994: 58-61.
[7] 张闿. 捕收剂混合使用的协同效应与其浮选性能的相关关系研究[J]. 矿冶工程, 1990(3):22-26+67.
ZHANG K. Study on the correlation between the synergistic effect of mixed use of collectors and its flotation performance[J]. Mining and Metallurgical Engineering, 1990(3):22-26+67.
[8] 李如, 于良民, 闫雪峰, 等. Cu2O/ZnO的形貌可控制备及光催化性能[J]. 高等学校化学学报, 2017, 38(2):267-274.
LI R, YU L M, YAN X F, et al. Morphology-controlled preparation and photocatalytic Properties of Cu2O/ZnO microstructures[J]. Chemical Journal of Chinese Universities, 2017, 38(2):267-274.
[9] 张婉, 唐婉莹, 何世颖, 等. ZnO-PMPP复合材料光催化去除水中低浓度氨氮[J]. 环境科学学报, 2017, 37(2):267-274.
ZHANG W, TANG W Y, HE S Y, et al. Synthesis of ZnO-PMPP composites for photocatalytic removal of ammonia nitrogen at low concentration[J]. Acta Scientiae Circumstantiae, 2017, 37(2):267-274.
[10] 赵晓华, 苏帅, 武广利, 等. 片花状ZnO@碳球核壳结构的制备及太阳光催化性能[J]. 无机化学学报, 2017, 33(2):276-284.
ZHAO X H, SU S, WU G L, et al. Preparation and sunlight photocatalytic performance of flower-like ZnO@carbon sphere core-shell structure[J]. Chinese Journal of Inorgnic Chemistry, 2017, 33(2):276-284.
-



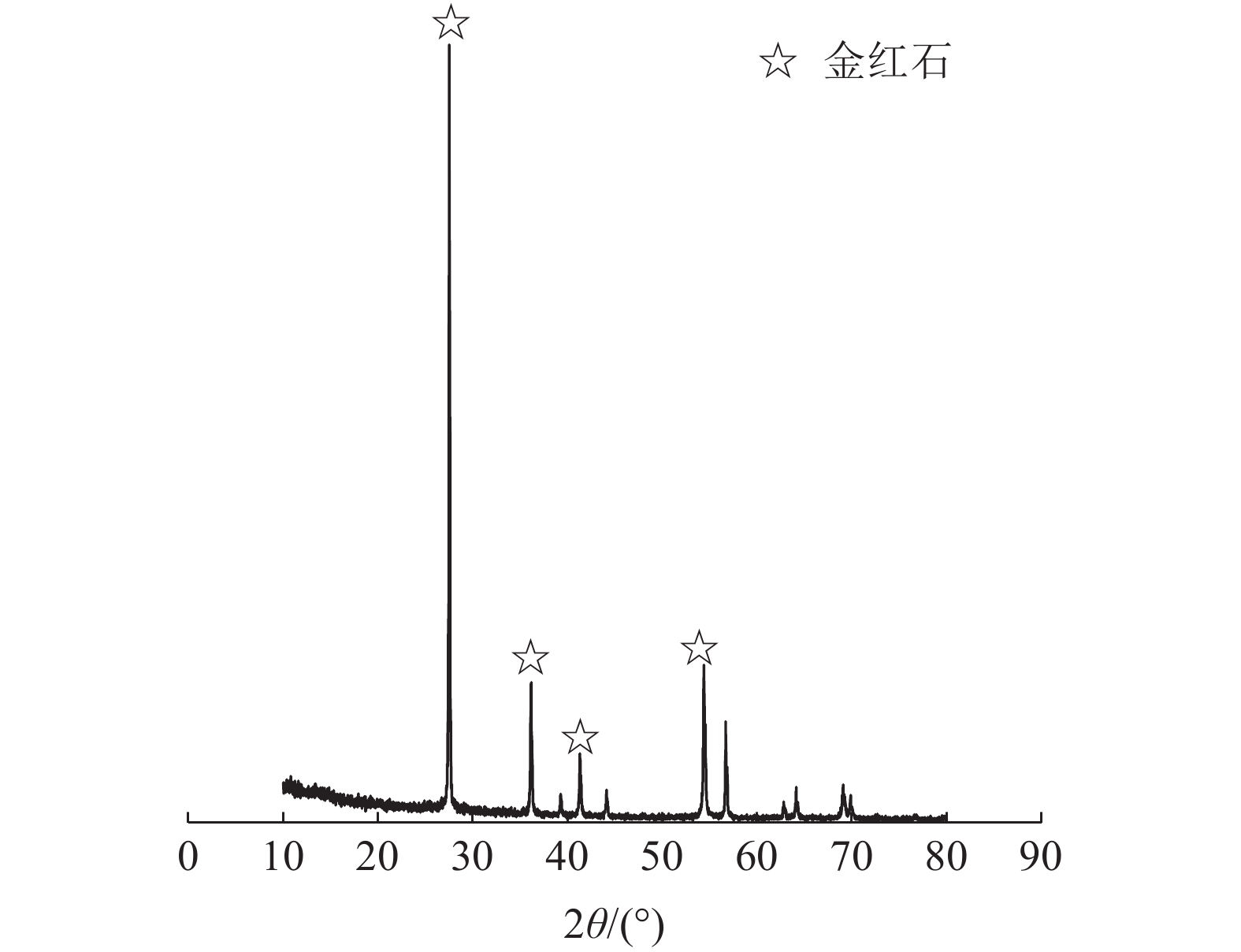
 下载:
下载:




