Thermodynamics Analysis on Hydrochloric Acid Leaching for Waste Ceria-Based Rare Earth Polishing Powder
-
摘要:
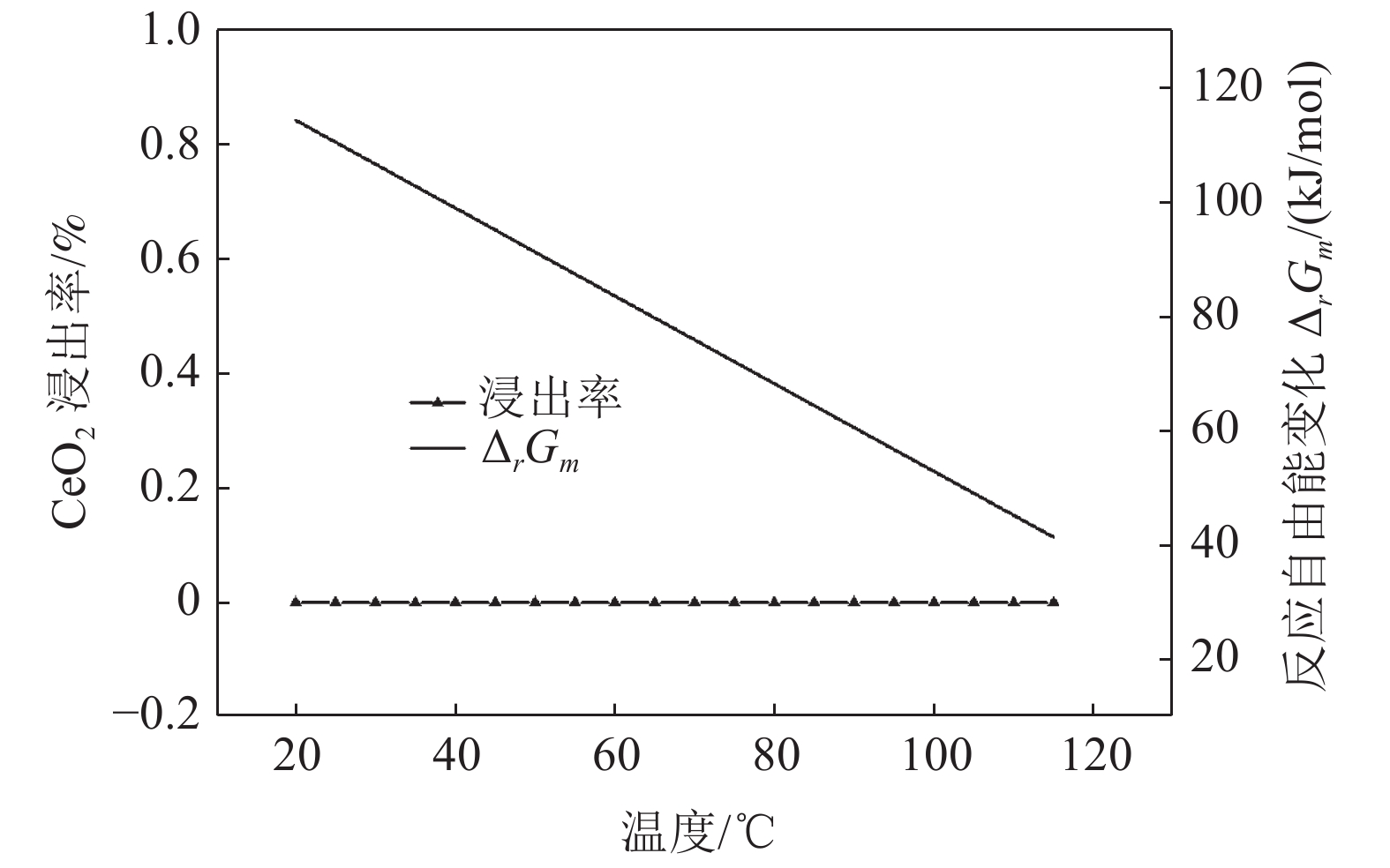
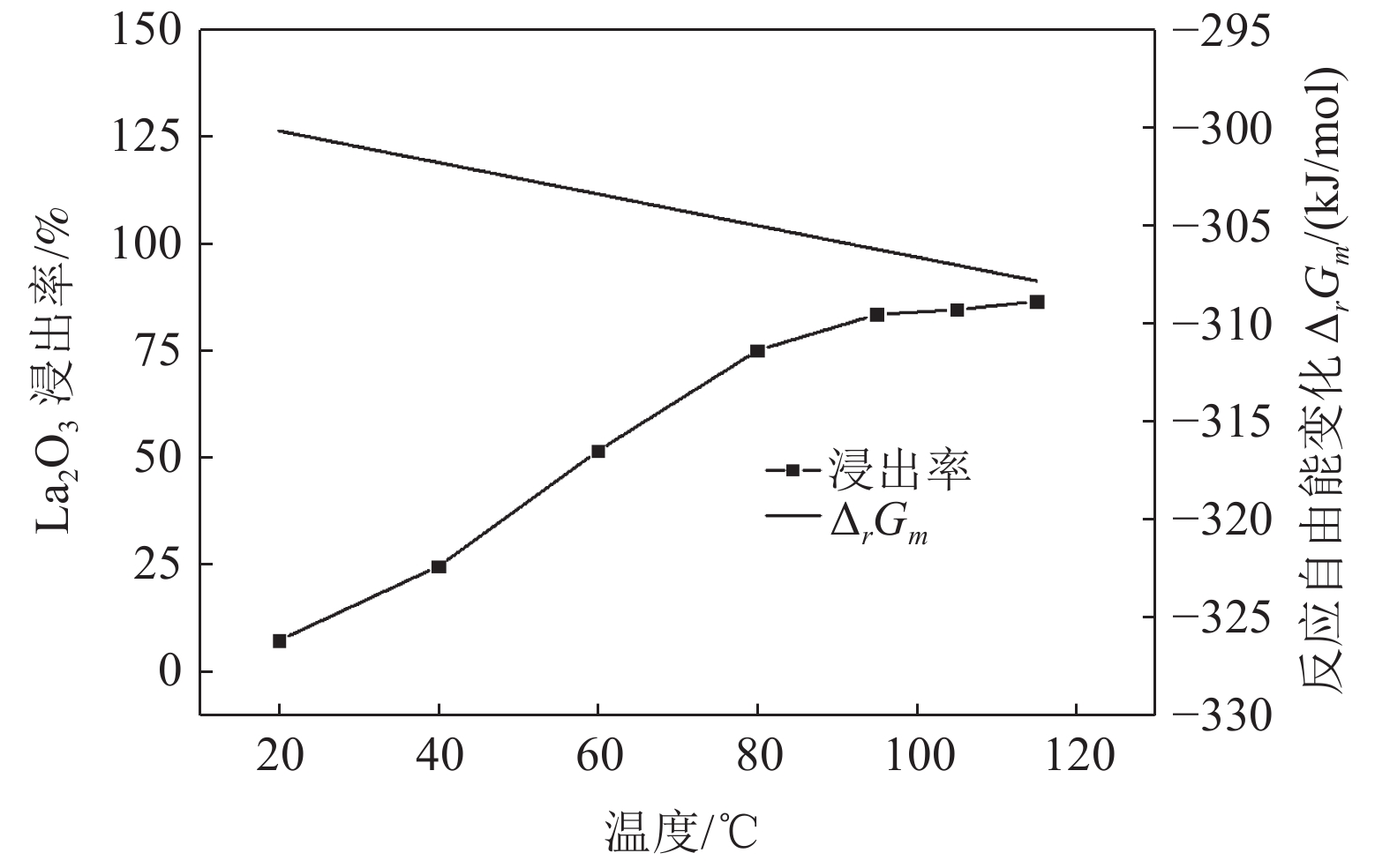
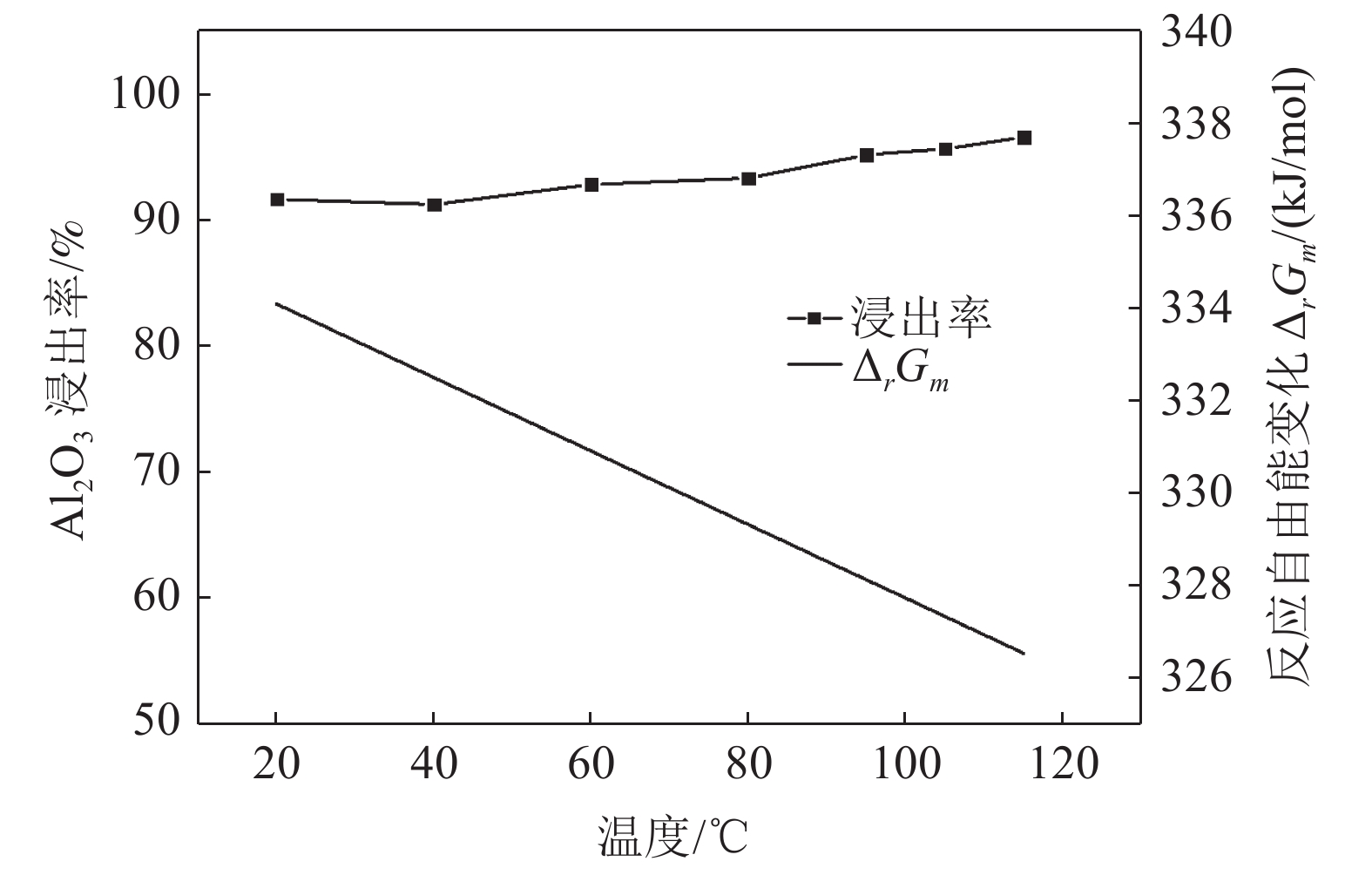
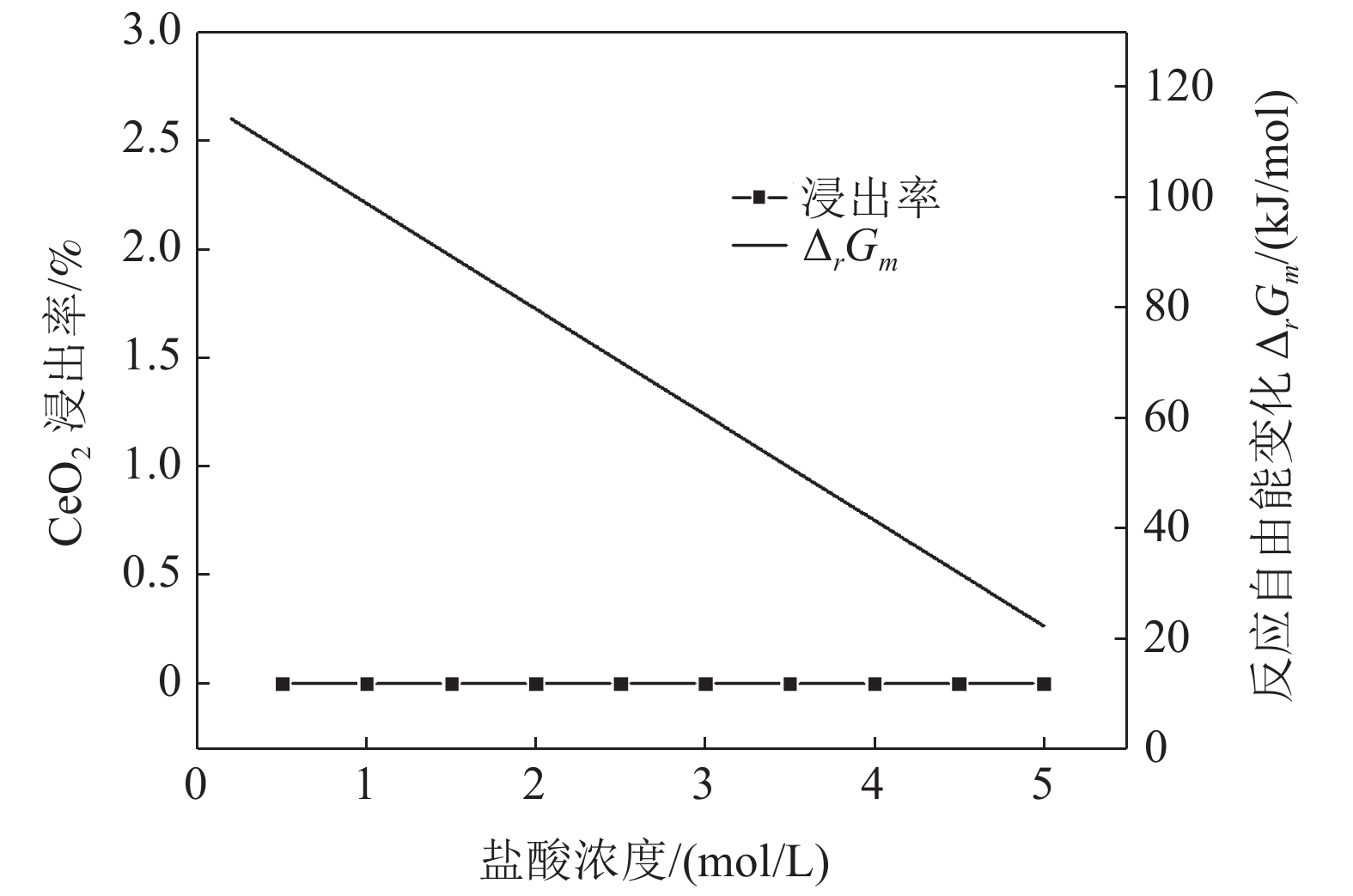
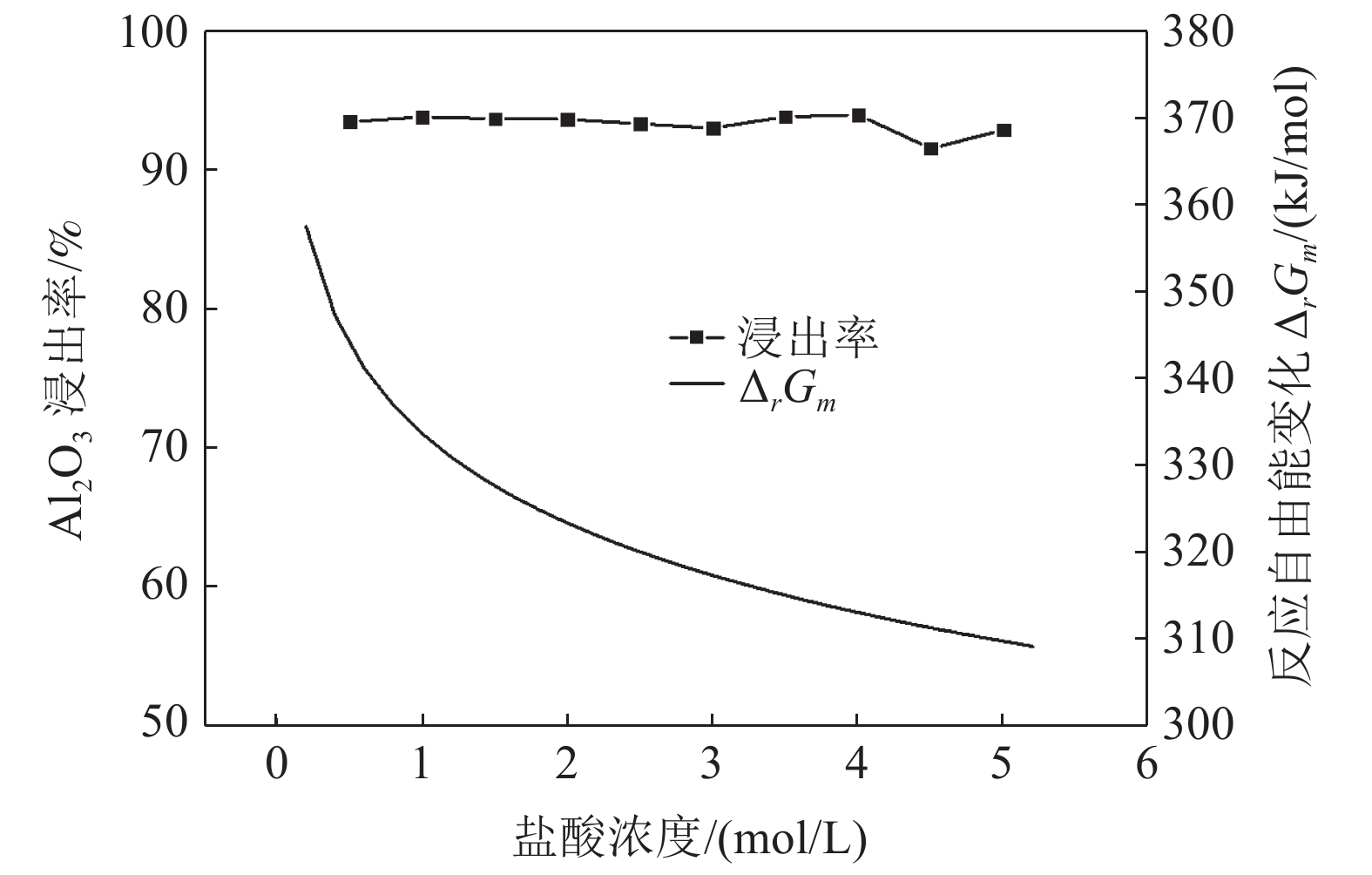
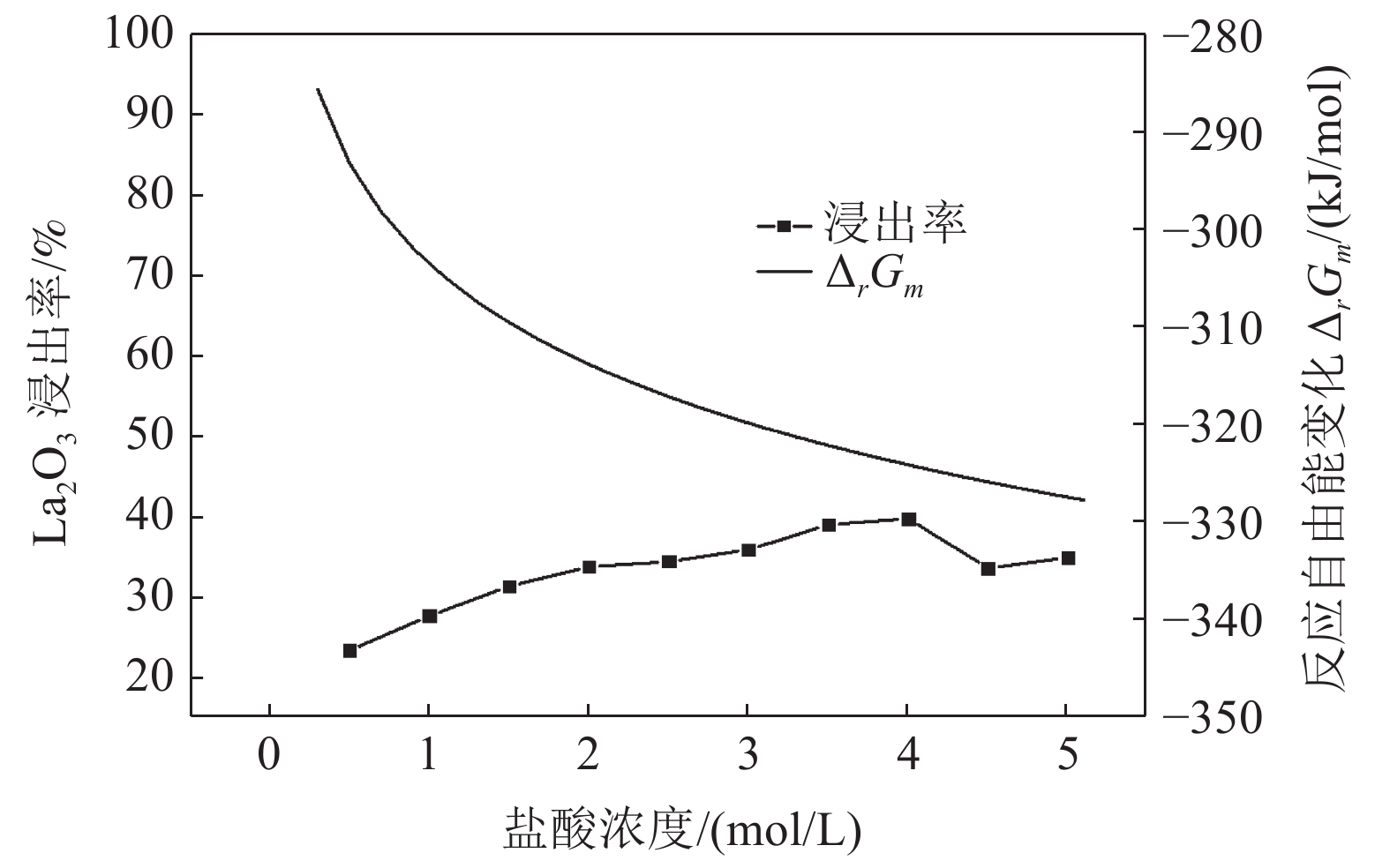
这是一篇冶金工程领域的论文。采用反应热力学方法研究盐酸浸出铈基稀土抛光粉废料的浸出反应,主要考虑浸出温度和盐酸浓度两个影响反应热力学参数,结合盐酸浸出实验结果和浸出反应的Gibbs自由能理论计算,结果发现:抛光粉废料中的CeO2不与盐酸反应,La2O3能很容易被盐酸浸出溶解,实验结果和反应的Gibbs自由能理论计算相一致。然而,盐酸浸出抛光粉废料中的Al2O3,实验结果与理论分析完全相反,盐酸与Al2O3的反应Gibbs自由能大于零,理论上盐酸不能浸出该废料中的Al2O3,但实验结果表明Al2O3 的浸出率高于91%。这与废料中的Al2O3是反应活性高的非晶质的,而反应Gibbs自由能计算中采用稳定的Al2O3晶体结构(刚玉)的热力学参数所致。
Abstract:This is an essay in the field of metallurgical engineering. Thermodynamics of the reaction, hydrochloric acid leaching for waste ceria-based rare earth polishing powder, was studied in this work.The influences on the leaching rate of the waste polishing powder was respectively considered including hydrochloric acid concentration and leaching temperature in the experiment. The result of the Gibbs free energy of leaching reaction and leaching experiment showed, thermodynamics theoretical analysis was consistent with experiment date for CeO2 and La2O3. CeO2 could not be immersed in dissolution, and La2O3 was easy to get leached. However, the experiment result was quite opposite of theoretical thermodynamics calculation for Al2O3. The Gibbs free energy of leaching reaction for Al2O3 were always a positive value, and Al2O3 from the waste polishing powder couldn’t be immersed by hydrochloric acid, but experiment result indicated that the Al2O3 leaching rate can be stabilized above 91%. Amorphous forms Al2O3 had high reactive activity in the waste, but the thermodynamic parameter of inert Al2O3 crystal structure (corundum) was adopted in the Gibbs free energy calculation.
-

-
表 1 抛光粉废料的化学成分/%
Table 1. Chemical compositions of waste polishing power
CeO2 La3O2 Al2O3 SiO2 F CaO Fe2O3 Pr6O11 Nd2O3 53.36 19.07 18.52 7.68 4.7 1.77 1.03 0.88 0.13 表 2 物质的热力学数据[11]
Table 2. Thermodynamic parameters of substance
物质名称 化学式  /
/
(kJ/mol) /
/
(kJ/mol) /
/
(J/(K·mol)) /
/
(kJ/(K·mol))盐酸 HCl(l) -167.44 -131.17 55.10 氧化铈 CeO2(s) -1088.7 -1024.6 62.3 61.6 氧化镧 La2O3(s) -1793.7 -1705.8 127.3 108.8 氧化铝 Al2O3(s) -1675.7 -1582.3 50.9 79 氯化铈 CeCl3(aq) -1053.5 -977.8 151.0 87.4 氯化镧 LaCl3(aq) -1071.1 1028.99 108.8 氯化铝 AlCl3(aq) -704.2 -628.8 110.7 91.8 水 H2O(aq) -285.8 -237.1 70.0 75.3 氧气 O2(g) 0 0 205.2 29.4 -
[1] 罗天纵, 吴希桃, 包新军, 等. 废弃稀土抛光粉回收再利用研究进展[J]. 稀土, 2020, 41(3):95-104. LUO T Z, WU X T, BAO X J, et al. Research process in recovering and reutilizing of rare earth polishing powder wastes[J]. Chinese Rare Earths, 2020, 41(3):95-104. doi: 10.16533/J.CNKI.15-1099/TF.202003012
LUO T Z, WU X T, BAO X J, et al. Research process in recovering and reutilizing of rare earth polishing powder wastes[J]. Chinese Rare Earths, 2020, 41(3): 95-104. doi: 10.16533/J.CNKI.15-1099/TF.202003012
[2] 徐春涛, 李平辉, 李志锐, 等. 废弃稀土抛光粉再生利用的研究[J]. 稀土, 2017, 38(2):74-79. XU C T, LI P H, LI Z R, et al. Recovery of rare earth from waste polishing powder[J]. Chinese Rare Earths, 2017, 38(2):74-79. doi: 10.16533/J.CNKI.15-1099/TF.201702011
XU C T, LI P H, LI Z R, et al. Recovery of rare earth from waste polishing powder[J]. Chinese Rare Earths, 2017, 38(2): 74-79. doi: 10.16533/J.CNKI.15-1099/TF.201702011
[3] 黄娅琴, 付杰, 蒋昆, 等. 废弃稀土抛光粉的综合利用综述[J]. 资源节约与环保, 2018(1):70-79. HUANG Y Q, FU J, JIANG K, et al. Summary of the comprehensive utilization form rare earth polishing powder waste[J]. Resource Conservation and Environmental Protection, 2018(1):70-79. doi: 10.3969/j.issn.1673-2251.2018.01.046
HUANG Y Q, FU J, JIANG K, et al. Summary of the comprehensive utilization form rare earth polishing powder waste[J]. Resource Conservation and Environmental Protection, 2018(1): 70-79. doi: 10.3969/j.issn.1673-2251.2018.01.046
[4] 许涛, 于亚辉, 吕保义, 等. 稀土抛光粉固体废粉资源特性研究[J]. 中国资源综合利用, 2010, 28(5):22-25. XU T, YU Y H, LV B Y, et al. Research on resource characteristic of solid waste RE polishing powder[J]. China Resources Comprehensive Utilization, 2010, 28(5):22-25. doi: 10.3969/j.issn.1008-9500.2010.05.020
XU T, YU Y H, LV B Y, et al. Research on resource characteristic of solid waste RE polishing powder[J]. China Resources Comprehensive Utilization, 2010, 28(5): 22-25. doi: 10.3969/j.issn.1008-9500.2010.05.020
[5] WANG Li-Pang, CHEN Yan-Jiang, TSO Yun-Chen. Separation of cerium oxide abrasive and glass powder in an abrasive-glass polishing waste by means of liquid-liquid-powder extraction method for recovery: acomparison of using a cationic andan anionic surfactant collector[J]. Sustainability, 2020, 12(11):1-13.
[6] 赵文怡, 孟志军, 刘海蛟, 等. 废抛光粉中稀土的回收[J]. 稀土, 2012, 33(6):75-78. ZHAO W Y, MENG Z J, LIU H J, et al. Recovery of rare earth from waste polishingpowder[J]. Chinese Rare Earths, 2012, 33(6):75-78. doi: 10.16533/j.cnki.15-1099/tf.2012.06.029
ZHAO W Y, MENG Z J, LIU H J, et al. Recovery of rare earth from waste polishingpowder[J]. Chinese Rare Earths, 2012, 33(6): 75-78. doi: 10.16533/j.cnki.15-1099/tf.2012.06.029
[7] 伍莺, 陈冬英, 欧阳红, 等. 从稀土抛光粉废料中回收稀土试验研究[J]. 湿法冶金, 2015, 34(5):398-401. WU Y, CHEN D Y, OUYANG H, et al. Recovery of rare earth from waste polishing powders[J]. Hydrometallurgy of China, 2015, 34(5):398-401. doi: 10.13355/j.cnki.sfyj.2015.05.011
WU Y, CHEN D Y, OUYANG H, et al. Recovery of rare earth from waste polishing powders[J]. Hydrometallurgy of China, 2015, 34(5): 398-401. doi: 10.13355/j.cnki.sfyj.2015.05.011
[8] 杨庆山, 谢圣中, 徐拓, 等. 稀土抛光粉废料再生利用试验研究[J]. 稀有金属与硬质合金, 2018, 46(5):43-45. YANG Q S, XIE S Z, XU T, et al. Experimental study on reutilization of waste rare earth polishing powder[J]. Rare Metals and Cemented Carbides, 2018, 46(5):43-45.
YANG Q S, XIE S Z, XU T, et al. Experimental study on reutilization of waste rare earth polishing powder[J]. Rare Metals and Cemented Carbides, 2018, 46(5): 43-45.
[9] BAO Xin-jun, ZHANG Ze-jie, LUO, Tian-zhong, et al. Conversion of cerium and lanthanum from rare earth polishing powder wastes to CeO2 and La0.6Ca0.4CoO3[J]. Hydrometallurgy, 2020, 193:1-10.
[10] WANG Li-Pang, LIU Pei-Hsin, CHEN Yan-Jiang. Recovery of cerium oxide abrasive from an abrasive-glass polishing waste through alkaline roasting followed by water leaching[J]. Metals, 2020, 10(6):1-15.
[11] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993.
LIANG Y J, CHE Y C. The inorganic thermodynamics data manual[M]. Shenyang: Northeastern University Press, 1993.
-



 下载:
下载: