Experimental on the Adsorption of Kaolin to Wastewater Containing Cu2+ and Pb2+
-
摘要:
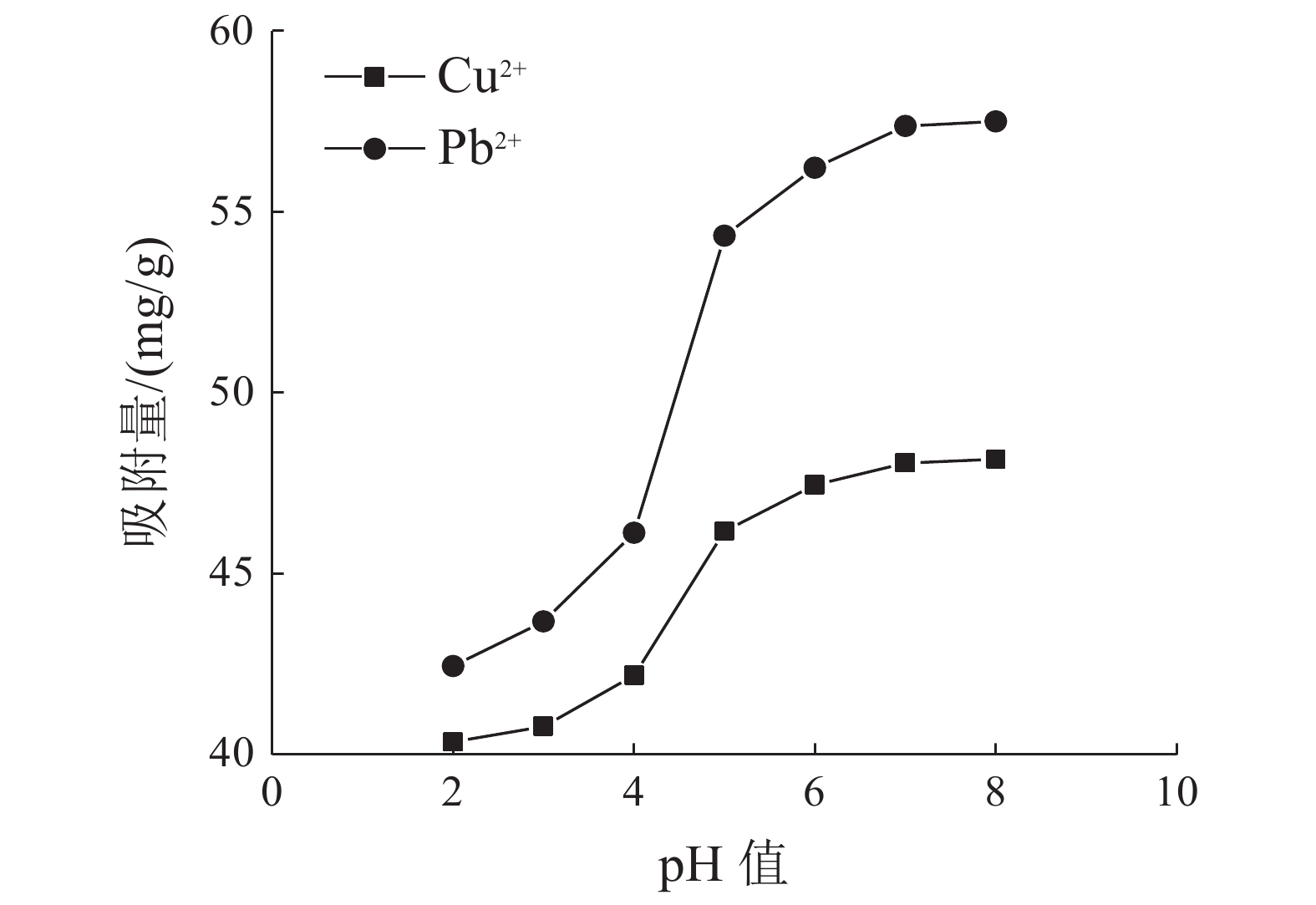
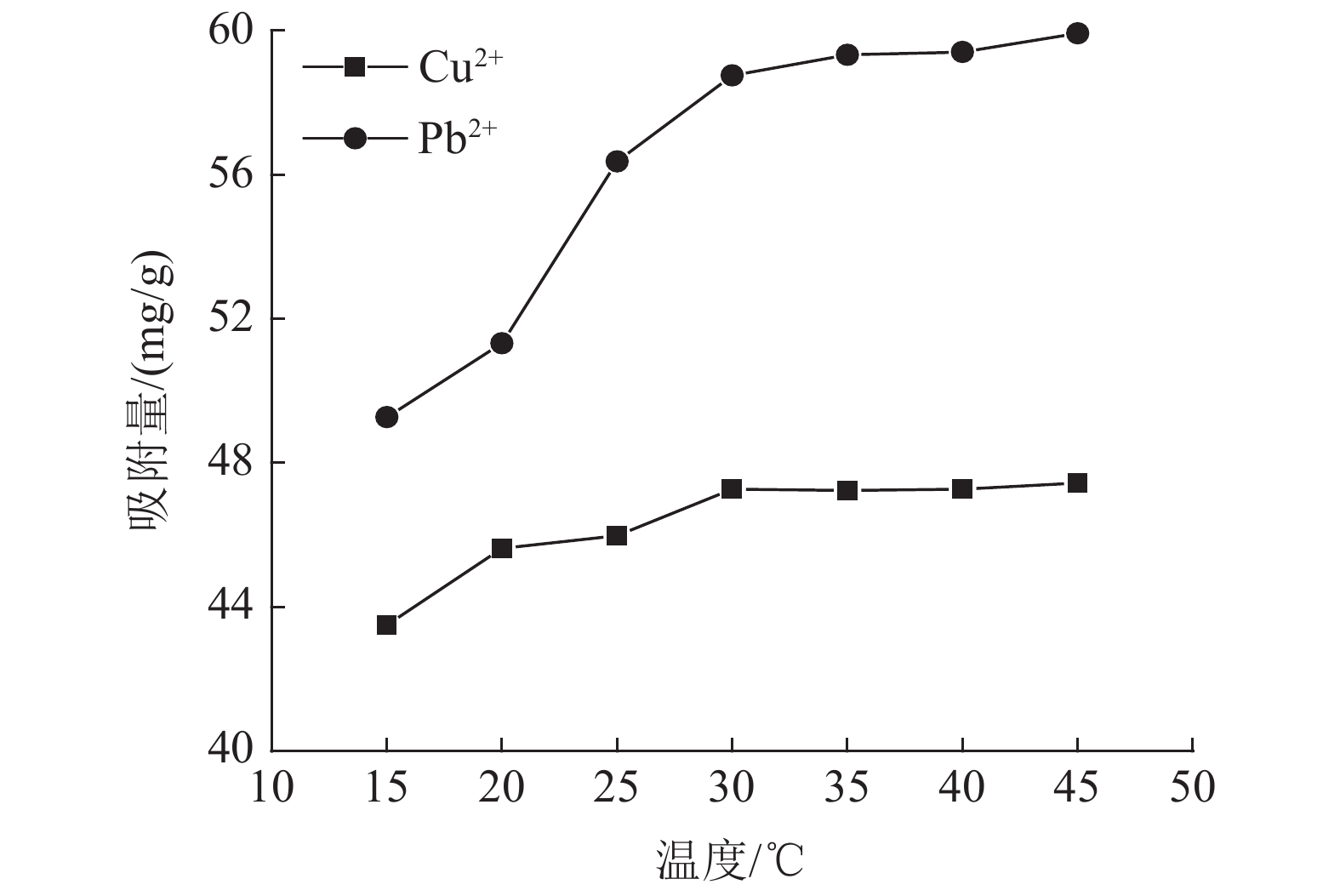
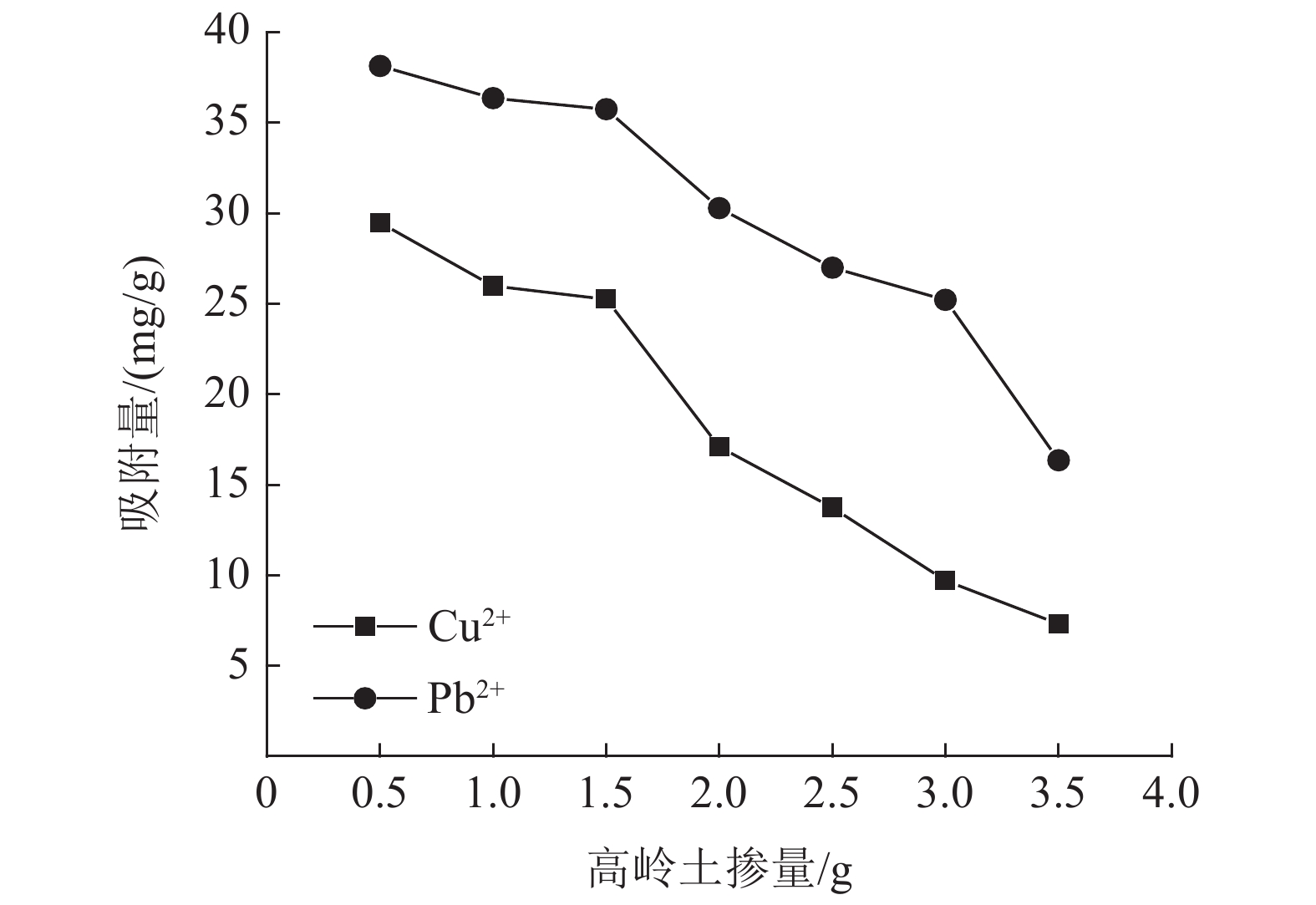
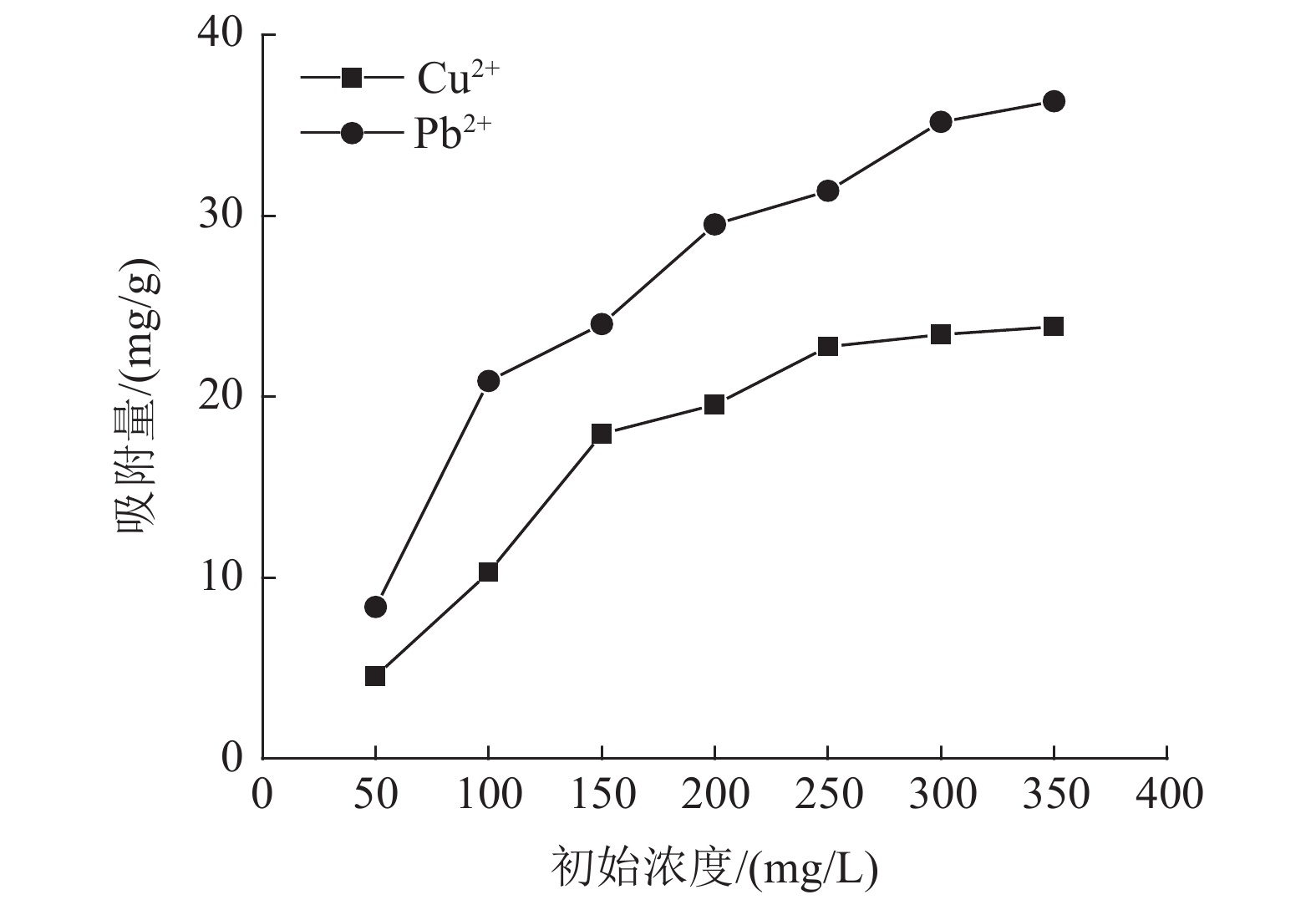
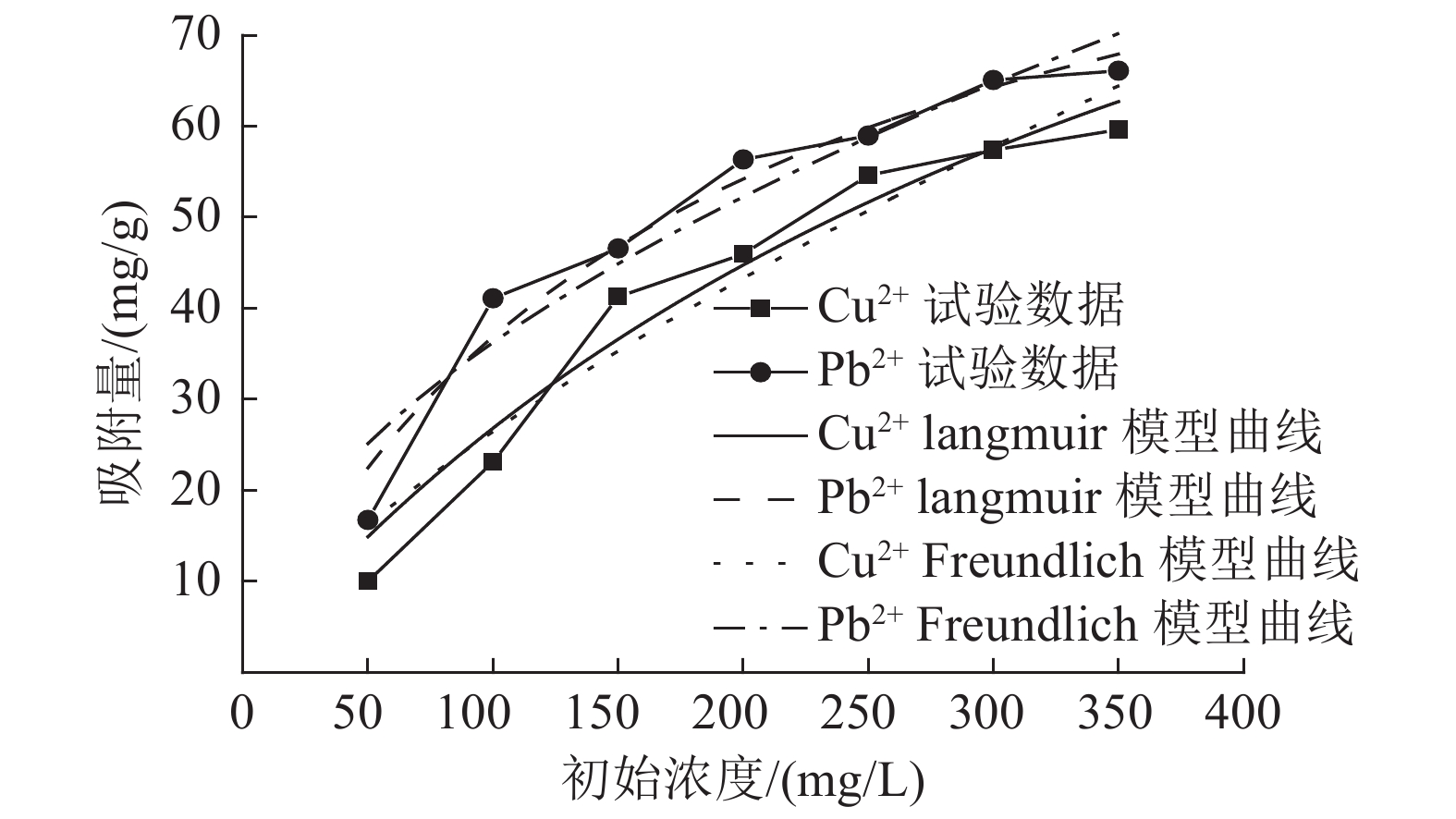
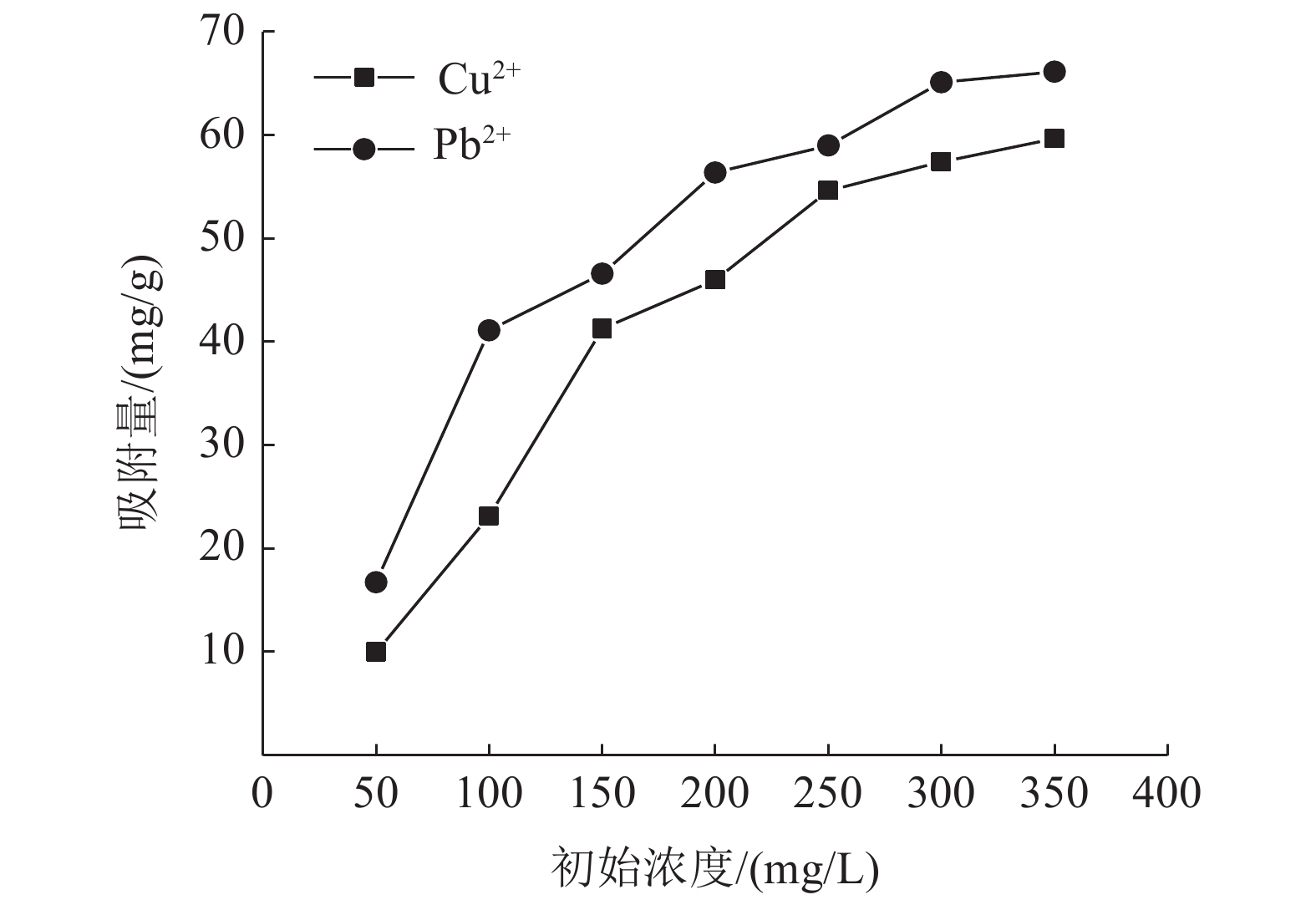
这是一篇环境工程领域的论文。为了研究高岭土对含有Cu2+和Pb2+污水吸附性能的影响,开展了不同初始浓度、温度、吸附时间、高岭土掺量和pH值作用下高岭土吸附重金属离子实验,并分析了高岭土对Cu2+和Pb2+共同吸附实验结果。结果表明:高岭土吸附金属Pb2+离子的效果要好于高岭土吸附金属Cu2+离子的效果。结合实验结果和经济效益而言,在初始浓度为200 mg/L,pH值为6、温度为30 ℃,高岭土掺量为1.5 g,吸附时间为2.0 h时,高岭土对金属Pb2+和Cu2+离子的吸附效果较优,其中金属Pb2+离子的吸附量分别达到了56.38、56.22、58.76、35.75、和42.42 mg/g,金属Cu2+离子的吸附量45.99、47.45、47.27、25.26、22.52 mg/g。整体上,高岭土对共同吸附金属离子(Cu2+、Pb2+)的吸附量要小于单一离子的吸附量,这是由于两个金属离子在吸附过程中会相互影响对方的吸附过程。Langmuir模型对实验曲线的拟合度要远远高于Freundlich模型对实验曲线的拟合度,这就说明了Langmuir等温吸附模型更加适用于高岭土吸附金属离子吸附量的变化规律,进而证明了高岭土吸附重金属离子属于表面吸附,被吸附的重金属离子都是相互独立存在的。
Abstract:This is an article in the field of environmental engineering. In order to study the effect of kaolin on the adsorption performance of wastewater containing Cu2+ and Pb2+, experiments on the adsorption of heavy metal ions by kaolin under different initial concentrations, temperatures, adsorption time, kaolin content and pH values are carried out. And analyzed the results of Cu2+ and Pb2+ co-adsorption test by kaolin. The results show that the adsorption effect of kaolin on metal Pb2+ ions is better than the adsorption effect of kaolin on metal Cu2+ ions. Combining the test results and economic benefits, when the initial concentration is 200 mg/L, the pH is 6, the temperature is 30 °C, the kaolin content is 1.5 g, and the adsorption time is 2.0 h, Kaolin has the best adsorption effect on metal Pb2+ and Cu2+ ions. The adsorption capacity of metal Pb2+ ions reached 56.38, 56.22, 58.76, 35.75, and 42.42 mg/g, respectively. The adsorption capacity of metallic Cu2+ ions is 45.99, 47.45, 47.27, 25.26, 22.52 mg/g. On the whole, the adsorption capacity of kaolin for co-adsorbed metal ions (Cu2+, Pb2+) is smaller than that of a single ion. This is because two metal ions will affect each other's adsorption process during the adsorption process. The fit of Langmuir model to the test curve is much higher than the fit of Freundlich model to the test curve. This shows that the Langmuir isotherm adsorption model is more suitable for the change law of the amount of metal ions adsorbed by kaolin. Furthermore, it is proved that the adsorption of heavy metal ions by kaolin belongs to surface adsorption, and the adsorbed heavy metal ions exist independently of each other.
-

-
表 1 Langmuir和Freundlich模型参数
Table 1. Langmuir and Freundlich model parameters
金属离子 Freundlich模型 Langmuir模型 Cu2+ KF n R2 K Qmax R2 1.006 0.710 0.925 0.002 134.941 0.954 Pb2+ KF n R2 K Qmax R2 3.194 0.528 0.913 0.006 102.461 0.960 -
[1] 许斌, 韩萍, 薛玉芬. 污水厂中草甘膦降解菌的筛选及其降解特性研究[J]. 中国农学通报, 2021, 37(14): 84-89.XU B, HAN P, XUE Y F. Screening of glyphosate-degrading bacteria in wastewater plants and their degradation characteristics[J]. Chinese Agronomy Bulletin, 2021, 3 7(14): 84-89.
XU B, HAN P, XUE Y F. Screening of glyphosate-degrading bacteria in wastewater plants and their degradation characteristics[J]. Chinese Agronomy Bulletin, 2021, 3 7(14): 84-89. [2] 闫英师, 李玉凤, 赵礼兵. 改性钢渣吸附重金属离子的研究现状[J]. 矿产综合利用, 2021(1):8-13.YAN Y S, LI Y F, ZHAO L B. Research status of heavy metal ions adsorption by modified steel slag[J]. Multipurpose Utilization of Mineral Resources, 2021(1):8-13 doi: 10.3969/j.issn.1000-6532.2021.01.002
YAN Y S, LI Y F, ZHAO L B . Research status of heavy metal ions adsorption by modified steel slag[J]. Multipurpose Utilization of Mineral Resources,2021 (1 ):8 -13 [3] 胡超, 包惠明, 迟恩涛, 等. 高岭土尾矿沥青混合料抗腐性能实验与机理研究[J]. 矿产综合利用, 2020(5):161-168.HU C, BAO H M, CHI E T, et al. Test and mechanism study on corrosion resistance of kaolin tailings asphalt mixture[J]. Multipurpose Utilization of Mineral Resources, 2020(5):161-168. doi: 10.3969/j.issn.1000-6532.2020.05.026
HU C, BAO H M, CHI E T, et al . Test and mechanism study on corrosion resistance of kaolin tailings asphalt mixture[J]. Multipurpose Utilization of Mineral Resources,2020 (5 ):161 -168 .[4] 罗宿星, 陈华仕, 牟青松, 等. 黄铁矿的吸附性能研究现状及进展[J]. 矿产综合利用, 2020(5):26-33.LUO S X, CHEN H S, MOU Q S, et al. Research situation and progress of adsorption properties of pyrite[J]. Multipurpose Utilization of Mineral Resources, 2020(5):26-33.
LUO S X, CHEN H S, MOU Q S, et al . Research situation and progress of adsorption properties of pyrite[J]. Multipurpose Utilization of Mineral Resources,2020 (5 ):26 -33 .[5] 孔贇, 田焜, 王月兰, 等. 改性高岭土尾矿复合水晶废渣在水泥基材料中的应用研究[J]. 混凝土与水泥制品, 2018(10): 103-106.KONG Y, TIAN K, WANG Y L , et al. Research on the application of modified kaolin tailings composite crystal waste in cementitious materials[J]. Concrete and Cement Products, 2018(10): 103-106.
KONG Y, TIAN K, WANG Y L , et al. Research on the application of modified kaolin tailings composite crystal waste in cementitious materials[J]. Concrete and Cement Products, 2018(10): 103-106. [6] 曾琴, 周义朋, 黎广荣, 等. 高岭土对酸性水中铀的吸附试验[J]. 有色金属(冶炼部分), 2021(5):97-102.ZENG Q, ZHOU Y P, LI G R, et al. Adsorption test of kaolin on uranium in acidic water[J]. Nonferrous Metals(Extractive Metallurgy ), 2021(5):97-102.
ZENG Q, ZHOU Y P, LI G R, et al . Adsorption test of kaolin on uranium in acidic water[J]. Nonferrous Metals(Extractive Metallurgy ),2021 (5 ):97 -102 .[7] 祝雯霞, 张其武, 李学伟, 等. 活化蛇纹石促进磷酸改性高岭土对K+的吸附试验研究[J]. 金属矿山, 2020(11):134-140.ZHU M X, ZHANG Q W, LI X W, et al. Experimental study on the adsorption of K+ by activated serpentine to promote phosphate-modified kaolin[J]. Metal Mining, 2020(11):134-140.
ZHU M X, ZHANG Q W, LI X W, et al . Experimental study on the adsorption of K+ by activated serpentine to promote phosphate-modified kaolin[J]. Metal Mining,2020 (11 ):134 -140 .[8] 左继超, 胡红青, 刘永红, 等. 磷和柠檬酸共存对高岭石和针铁矿吸附铅的影响[J]. 土壤学报, 2017, 54(1):265-272.ZUO J C, HU H Q, LIU Y H, et al. Effect of coexistence of phosphorus and citric acid on the adsorption of lead by kaolinite and clinoptilolite[J]. Soil Journal, 2017, 54(1):265-272.
ZUO J C, HU H Q, LIU Y H, et al . Effect of coexistence of phosphorus and citric acid on the adsorption of lead by kaolinite and clinoptilolite[J]. Soil Journal,2017 ,54 (1 ):265 -272 .[9] 黄亮国, 朱燕娟, 赵韦人, 等. 高岭土最佳分散条件的确定与探讨[J]. 中国造纸, 2009, 28(6):18-21.HUANG L G, ZHU Y J, ZHAO W R, et al. Determination and exploration of optimal dispersion conditions of kaolin[J]. China Paper Making, 2009, 28(6):18-21.
HUANG L G, ZHU Y J, ZHAO W R, et al . Determination and exploration of optimal dispersion conditions of kaolin[J]. China Paper Making,2009 ,28 (6 ):18 -21 .[10] 王曼曼, 石林, 张洋洋. 伊利石合成沸石相吸附材料及对水中Ni2+的吸附[J]. 矿产综合利用, 2021(2):192-198.WANG M M, SHI L, ZHANG Y Y. Adsorption of Ni2+ from aqueous solutions by zeolite phase adsorption materials synthesized from illite[J]. Multipurpose Utilization of Mineral Resources, 2021(2):192-198.
WANG M M, SHI L, ZHANG Y Y . Adsorption of Ni2+ from aqueous solutions by zeolite phase adsorption materials synthesized from illite[J]. Multipurpose Utilization of Mineral Resources,2021 (2 ):192 -198 .[11] 袁建民. 粘土矿物对重金属离子的吸附能力研究[D]. 石家庄: 河北地质大学, 2018.YUAN J M. Research on the adsorption capacity of clay minerals on heavy metal ions[D]. Shijiazhuang: Hebei University of Geology, 2018.
YUAN J M. Research on the adsorption capacity of clay minerals on heavy metal ions[D]. Shijiazhuang: Hebei University of Geology, 2018. [12] Vilar V J , Botelho C M , Boaventura R A . Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour[J]. Journal of Hazardous Materials, 2007.
[13] 蒋明琴. 改性高岭土对废水中重金属离子的吸附性能研究[D]. 福州: 福建师范大学, 2009.JIANG M Q. Adsorption performance of modified kaolin on heavy metal ions in wastewater[D]. Fuzhou: Fujian Normal University, 2009.
JIANG M Q. Adsorption performance of modified kaolin on heavy metal ions in wastewater[D]. Fuzhou: Fujian Normal University, 2009. [14] 何宏平. 粘土矿物与金属离子作用研究[M]. 北京: 石油工业出版社, 2001.HE H P. Study on the interaction between clay minerals and metal ions [M]. Beijing: Petroleum Industry Press, 2001.
HE H P. Study on the interaction between clay minerals and metal ions [M]. Beijing: Petroleum Industry Press, 2001. [15] 王亚丽, 杨宁, 崔素萍, 等. 高炉渣对废水中Cu2+的吸附率和吸附行为[J]. 北京工业大学学报, 2021, 47(2):186-193.WANG Y L, YANG N, CUI S P, et al. Adsorption rate and adsorption behavior of blast furnace slag on Cu2+ in wastewater[J]. Journal of Beijing University of Technology, 2021, 47(2):186-193.
WANG Y L, YANG N, CUI S P, et al . Adsorption rate and adsorption behavior of blast furnace slag on Cu2+ in wastewater[J]. Journal of Beijing University of Technology,2021 ,47 (2 ):186 -193 . -



 下载:
下载: